Categories
Change Password!
Reset Password!


In patients infected with H. pylori, Ilaprazole-Amoxicillin dual therapy is equally effective, safer and less costly when compared to Ilaprazole-Amoxicillin-Furazolidone-Bismuth quadruple therapy.
The findings of an open-label randomized controlled clinical trial indicated that the Ilaprazole-Amoxicillin (IA) dual therapy at high doses is equally effective, safer, and more cost-effective compared to Ilaprazole-Amoxicillin-Furazolidone-Bismuth (IAFB) quadruple therapy for Helicobacter pylori (H. pylori) management. In this trial conducted among Chinese patients, researchers aimed to assess the effectiveness, safety, patient adherence, and cost-effectiveness of two different treatments for H. pylori infection.
The study involved 200 participants who had tested positive for H. pylori and were diagnosed with chronic gastritis. These patients were randomly assigned to two groups: Group A received a 14-day course of Ilaprazole-amoxicillin (IA) dual therapy (101 participants), while Group B received Ilaprazole-amoxicillin-furazolidone-bismuth (IAFB) quadruple therapy (99 participants). The efficacy of the treatments was assessed 4–6 weeks after completion using the 13C urea breath test.
A comparison of eradication rates, drug-associated adverse events, patient compliance, and drug costs was done between the two groups. Eradication rates were impressive in both groups, as shown in Table 1:
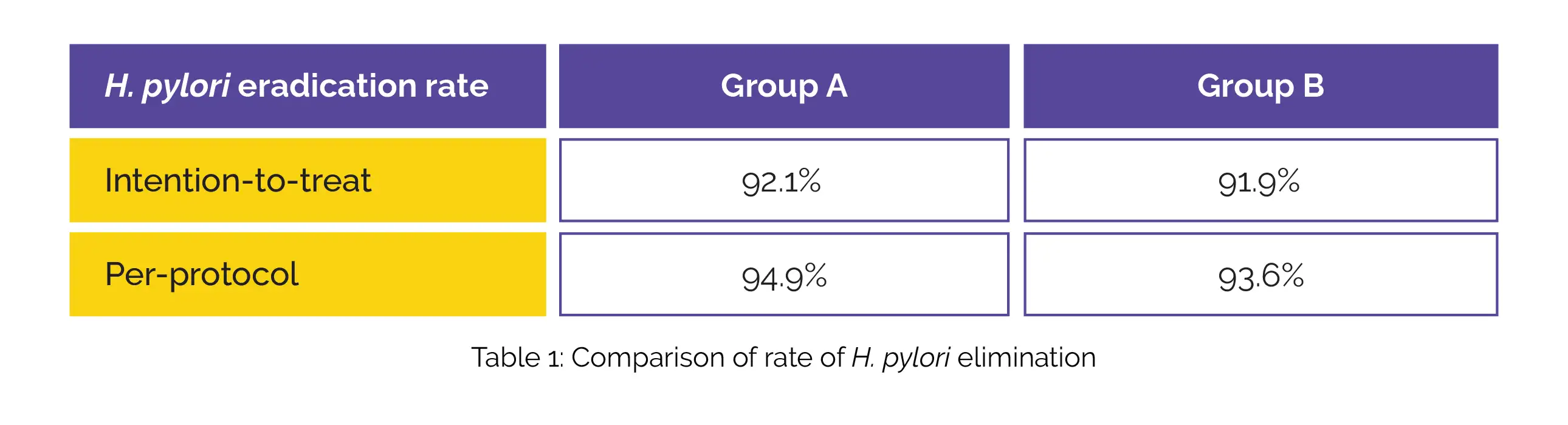
Furthermore, there was no vital difference in the occurrence of deleterious events between the treatment groups (P = 0.518). Notably, patient compliance was considerably greater in Group A in comparison with Group B (P = 0.031), and the cost of medication was also notably lower in Group A. Hence, these therapies could potentially serve as a first-line treatment option for H. pylori infection.
BMC Gastroenterology
Ilaprazole-amoxicillin dual therapy at high dose as a first-line treatment for helicobacter pylori infection in Hainan: a single-center, open-label, noninferiority, randomized controlled trial
Xiao-Dong Zhang et al.
Comments (0)