Categories
Change Password!
Reset Password!


Ferric carboxymaltose shows a notable reduction in iron deficiency anaemia throughout pregnancy and post-partum, with a faster haemoglobin increase compared to oral iron. However, this benefit might not endure post-delivery.
In comparison to oral iron, ferric carboxymaltose demonstrates a significant reduction in iron deficiency anaemia throughout pregnancy phase and into the post-partum period. It also offers a faster increase in haemoglobin concentration compared to oral iron. However, this advantage may not persist post-delivery, as deciphered from a study published in "The Lancet".
To address the alarming rates of anaemia affecting pregnant women in Africa, a team of researchers conducted an open-label, individually randomized controlled trial in Malawi. The study carried out focused on the use of a modern intravenous iron formulation called ferric carboxymaltose as a potential treatment for anaemia in pregnant women. The trial enrolled 862 women with singleton pregnancies, ranging from 13 to 26 weeks' gestation (second trimester), across primary care and outpatient settings. Subjects were included if they exhibited capillary haemoglobin levels of <10·0 g/dL and tested negative for malaria.
The women were randomly assigned to receive either a single dose of ferric carboxymaltose (up to 1000 mg) or the standard of care, which incorporated 60 mg elemental iron taken twice daily for 90 days, along with intermittent preventive malaria therapy. The key maternal outcome assessed was the prevalence of anaemia at 36 weeks' gestation, while the key neonatal outcome focused on birthweight. The results, analyzed in the intention-to-treat population, revealed that ferric carboxymaltose did not considerably minimize anaemia incidence at 36 weeks' gestation compared to the oral iron group (Table 1).
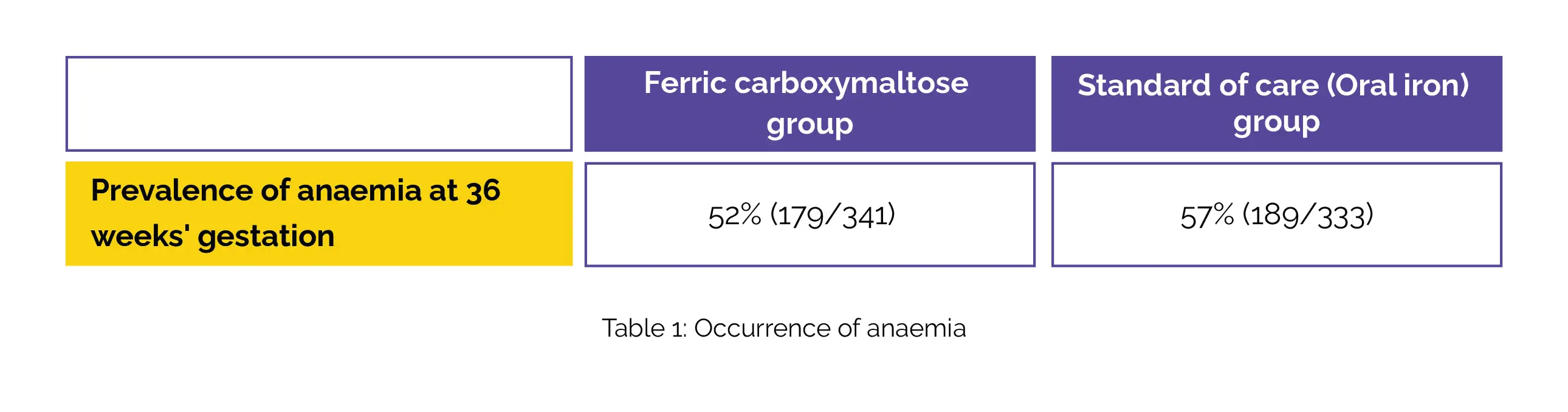
The prevalence of anaemia was numerically lower in the ferric carboxymaltose group at various time points, with statistical significance witnessed only at 4 weeks post-treatment. Birthweight, a crucial indicator of neonatal health, did not illustrate noticeable differences between the study groups (mean difference –3·1 g). The study reported no infusion-related serious adverse events, and the overall incidence of adverse events, including malaria, did not substantially differ between the two groups [ferric carboxymaltose-43% (183/430) vs standard of care-39% (170/432)].
The findings suggest that while ferric carboxymaltose treatment was deemed safe in this malaria-endemic sub-Saharan African setting, it did not demonstrate a substantial reduction in anaemia prevalence at 36 weeks' gestation or contribute to increased birthweight. This study underscores the challenges in addressing anaemia in pregnant women in the region and emphasizes the need for continued research and innovative interventions to improve maternal and neonatal health outcomes.
The Lancet
Ferric carboxymaltose versus standard-of-care oral iron to treat second-trimester anaemia in Malawian pregnant women: a randomised controlled trial
Sant-Rayn Pasricha et al.
Comments (0)