Categories
Change Password!
Reset Password!


In comparison with metronidazole-intensified triple therapy, bismuth-based quadruple therapy is highly cost-effective with similar efficacy in H. pylori patients.
In an open-label, multicenter, randomized controlled trial published in Gut Liver, a 14-day bismuth-based quadruple therapy (BQT) demonstrated comparable efficacy and more cost-effectiveness when compared to 14-day metronidazole-intensified triple therapy (MIT) for people diagnosed with clarithromycin-resistant Helicobacter pylori (H. pylori) infection with genotypic resistance.
Researchers undertook this study to assess the cost-effectiveness and efficacy of two therapies for the management of H. pylori infection. Utilizing sequencing-based clarithromycin resistance point mutation tests, examination of H. pylori-infected people was done. Overall, 782 volunteers with profound point mutations (A2144G, A2142C, A2143G, A2142G, A2143C) were randomized to get either MIT (n=99) or BQT (n=102).
The overall genotypic clarithromycin resistance rate was found to be 25.7%. The H. pylori elimination rate of BQT was not considerably distinct in the intention-to-treat analysis. However, it was remarkably greater when compared to MIT in the per-protocol analysis, as shown in Table 1:
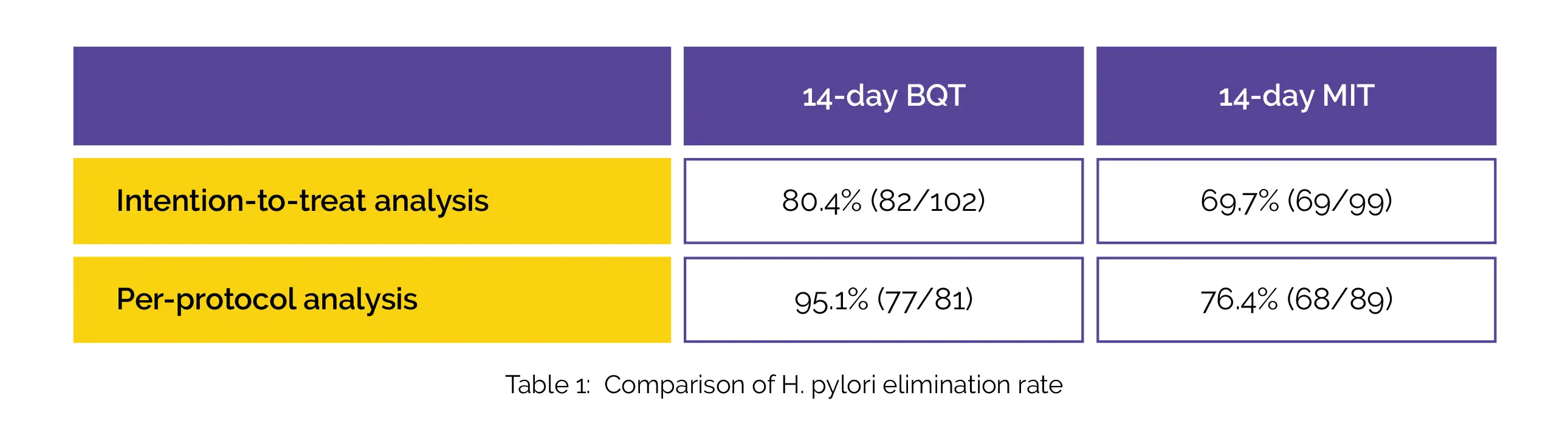
No profound differences were noted in the occurrence of adverse effects. Furthermore, compared to MIT, BQT was found to be more cost-effective. Thus, BQT and MIT show comparable effectiveness as first-line therapy for H. pylori infection with genotypic clarithromycin resistance.
Gut Liver
Bismuth-Based Quadruple Therapy versus Metronidazole-Intensified Triple Therapy as a First-Line Treatment for Clarithromycin-Resistant Helicobacter pylori Infection: A Multicenter Randomized Controlled Trial
Seung In Seo et al.
Comments (0)