Categories
Change Password!
Reset Password!


In hospitalized COVID-19 people, remdesivir was not linked with clinical improvement on day 15 or day 29.
According to the findings of phase 3 randomized controlled trial (DisCoVeRy), the utilization of remdesivir for the management of coronavirus-infected people was not related to clinical improvement on day 15 or day 29 when compared to the control group. Furthermore, remdesivir therapy did not decrease mortality and SARS-CoV-2 RNA. Investigators sought to find out the clinical efficacy of remdesivir in hospitalized adults suffering from SARS-CoV-2 hypoxemic pneumonia, with an indication of oxygen supplementation and/or ventilator support.
In this multicentre, open-label, parallel-group study, subjects were randomly segregated to get the usual standard of care alone or in combination with remdesivir, hydroxychloroquine, lopinavir/ritonavir, or lopinavir/ritonavir and interferon-β-1a. Remdesivir was given as 200 mg infusion on the first day. This was subsequently followed by once-daily infusions of 100 mg remdesivir up to nine days for ten days. It could be ceased after five days if the volunteer was discharged.
Clinical status at 15th day estimated by World Health Organization (WHO) 7-point ordinal scale, determined in intention-to-treat population was major endpoint ascertained. Overall, 857 subjects were randomized to one of the two groups and 843 subjects were incorporated for the final assessment (remdesivir, n=420; control, n=423). Regarding the occurrence of serious adverse events, no considerable inter-group difference was noted.
On day 15, distribution of WHO ordinal scale in the study groups is shown in Table 1:
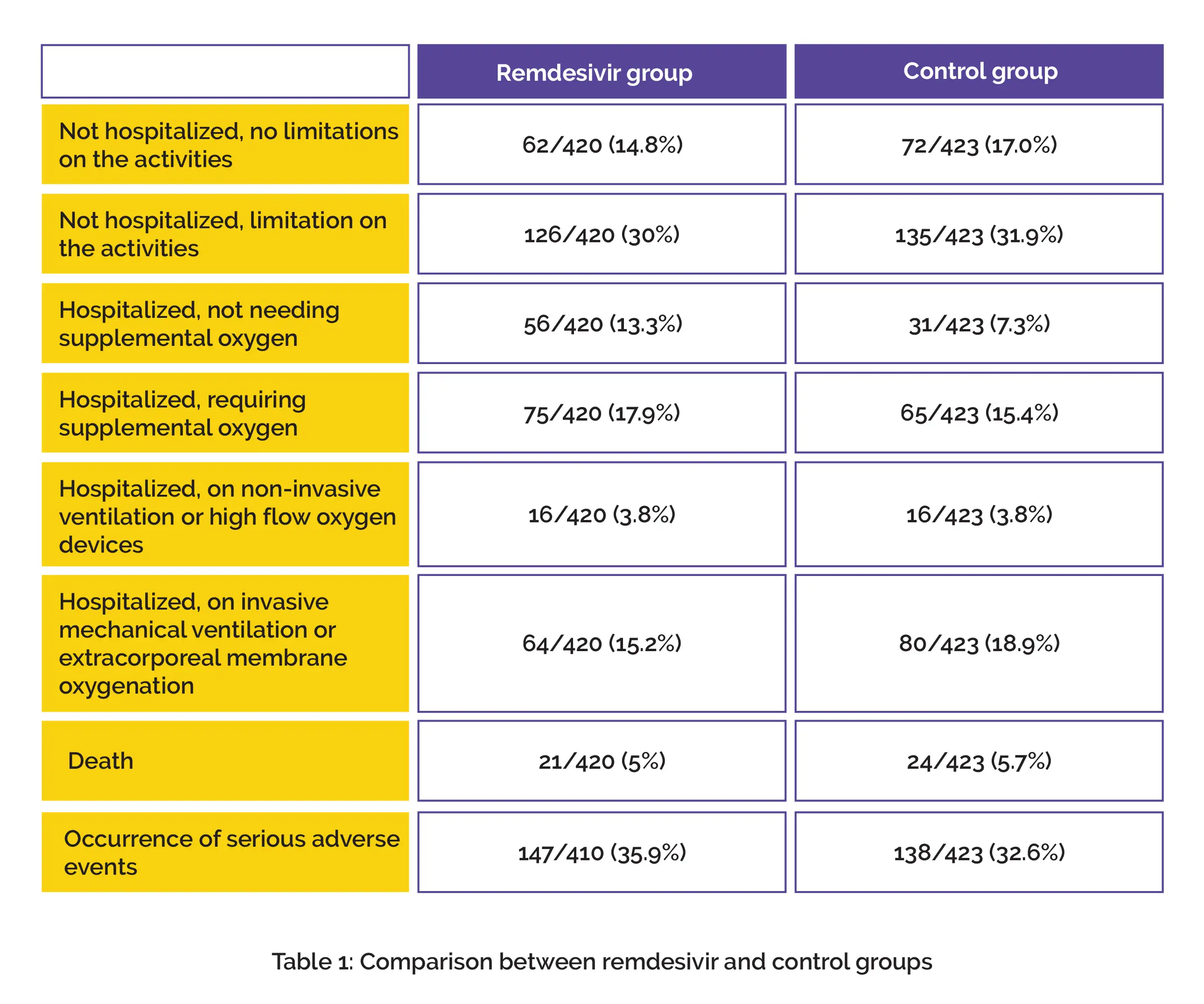
No profound difference was noted between the treatment groups (Odds ratio for remdesivir, 1.02). For the management of hospitalized people suffering from COVID-19, remdesivir use may not be beneficial.
medRxiv
Remdesivir for the treatment of hospitalised patients with COVID-19: final results from the DisCoVeRy randomised, controlled, open-label trial
Florence Ader et al.
Comments (0)