Categories
Change Password!
Reset Password!


The use of half a dose of Sofosbuvir routine in developing countries like Bangladesh can help to lower the cost burden in hepatitis C virus patients with end-stage renal disease.
The use of half-dose Sofosbuvir along with a full dose of Daclatasvir proved to be safe and cost-effective therapy for chronic hepatitis C people with end-stage renal disease (ESRD) on maintenance hemodialysis (MHD), the outcomes of a 3 years’ trial in a tertiary renal centre in Bangladesh elucidated. Sofosbuvir and Daclatasvir (directly acting antivirals) are highly useful to relieve hepatitis C virus (HCV) infection. This clinical trial by Masrura Jabin et al. assessed the effectiveness of half-dose Sofosbuvir in the management of HCV infections in ESRD patients on MHD.
On the whole, 125 HCV-positive patients with ESRD on MHD were included; all the patients underwent an HCV RNA polymerase chain reaction test. Based on this test, the patients were observed for 6 months to look for the occurrence of spontaneous viral clearance. After this period, the patients who had noticeable HCV-RNA were treated with Sofosbuvir 200 mg plus Daclatasvir dosed at 60 mg given every day for 12 weeks. A follow-up at 2 weeks was done for all the patients.
A periodic assessment of the values of total blood counts, liver function, and enzyme tests (creatinine phosphokinase and serum amylase) was done. The virological response was evaluated by HCV-RNA test after 12 weeks. About 23.2% (29 people) of total participants had spontaneous virus clearance with an undetectable HCV-RNA (Figure 1):
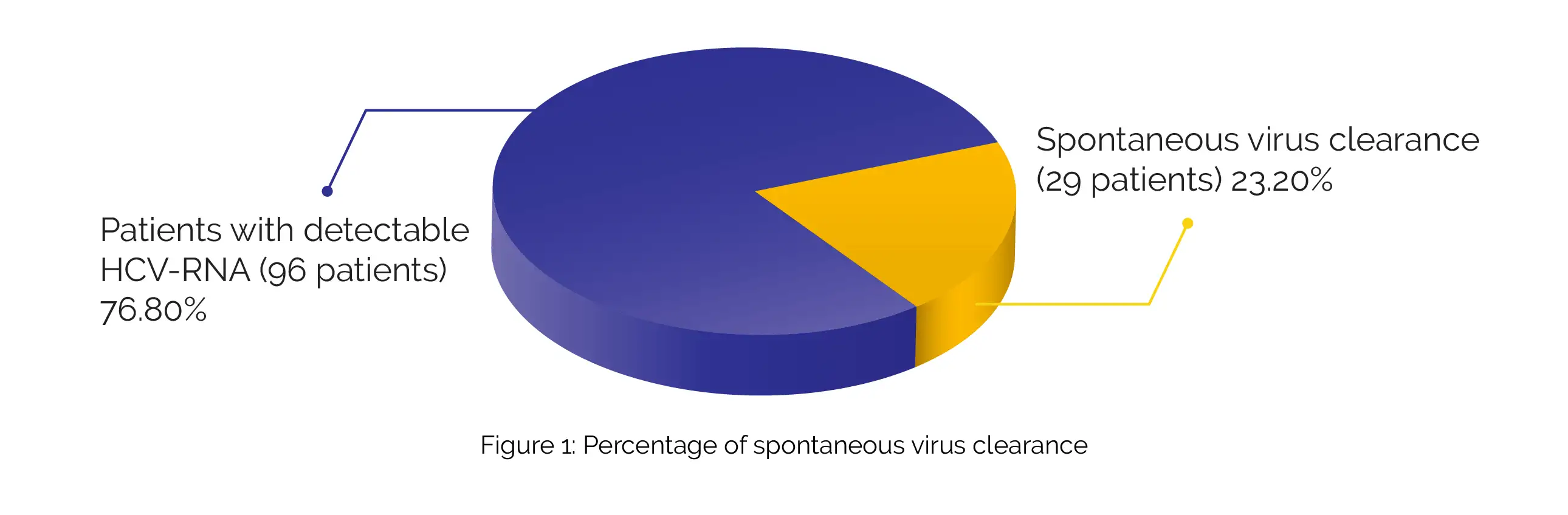
The average HCV-RNA level was 2.76 × 104 IU in the rest of the 96 patients. After a 12-week follow-up, 91 (94.8%) patients had a sustained virological response with undetectable HCV-RNA. There were no serious adverse events and study withdrawals signifying better tolerance for the therapy. The study authors repeated HCV-RNA in 30 patients who attained sustained virological response with antiviral drugs after 6 months.
In the majority of these patients (28, 93.3%), HCV-RNA were unnoticed whereas 2 patients (6.7%) had traces of viral infection again. The efficacy and safety of half-dose Sofosbuvir (200 mg after splitting the usual 400 mg into two halves) with Daclatasvir (60 mg) in patients with ESRD have been proven beneficial given the outcomes of this study.
Journal of Bangladesh College of Physicians and Surgeons
Efficacy of Daclatasvir and Half Dose Sofosbuvir in the Treatment of Hepatitis –C Virus Infection in Patients of Maintenance Hemodialysis-3 Years Trial in A Tertiary Renal Center
Masrura Jabin et al.
Comments (0)