Categories
Change Password!
Reset Password!


Phlai capsules, derived from Zingiber montanum, are safe and can ameliorate symptoms of allergic rhinitis.
In phase 3, randomized, double-blind, placebo-controlled study, Phlai capsules (Zingiber montanum, Ginger) were safe and led to modest improvements in the reflective total five symptom score (rT5SS), as well as reductions in itchy eyes, itchy nose, and rhinorrhea after a four-week treatment period. Investigators aimed to explore the safety and effectiveness of Phlai capsules as a treatment option for allergic rhinitis.
Patients diagnosed with allergic rhinitis were randomly assigned to 3 groups and administered either 100 mg or 200 mg of Zingiber montanum or a placebo once daily for a duration of 4 weeks. The alteration in the rT5SS was the major endpoint ascertained. Secondary outcomes included alterations in the peak nasal inspiratory flow (PNIF), Rhinoconjunctivitis Quality of Life-36 Questionnaire (RCQ-36) score, reflective individual symptom scores (itchy eyes, itchy nose, sneezing, nasal congestion, rhinorrhea), instantaneous total five symptom score (iT5SS), and the occurrence of any adverse events during the study period.
A total of 262 subjects were enrolled for the trial. Following 4 weeks of treatment, Zingiber montanum 100 mg demonstrated remarkable improvements in rT5SS (adjusted mean difference [aMD] -0.62) (Figure 1), as well as in itchy eyes (aMD -0.19), itchy nose (aMD -0.24), and rhinorrhea (aMD -0.19), compared to the placebo.
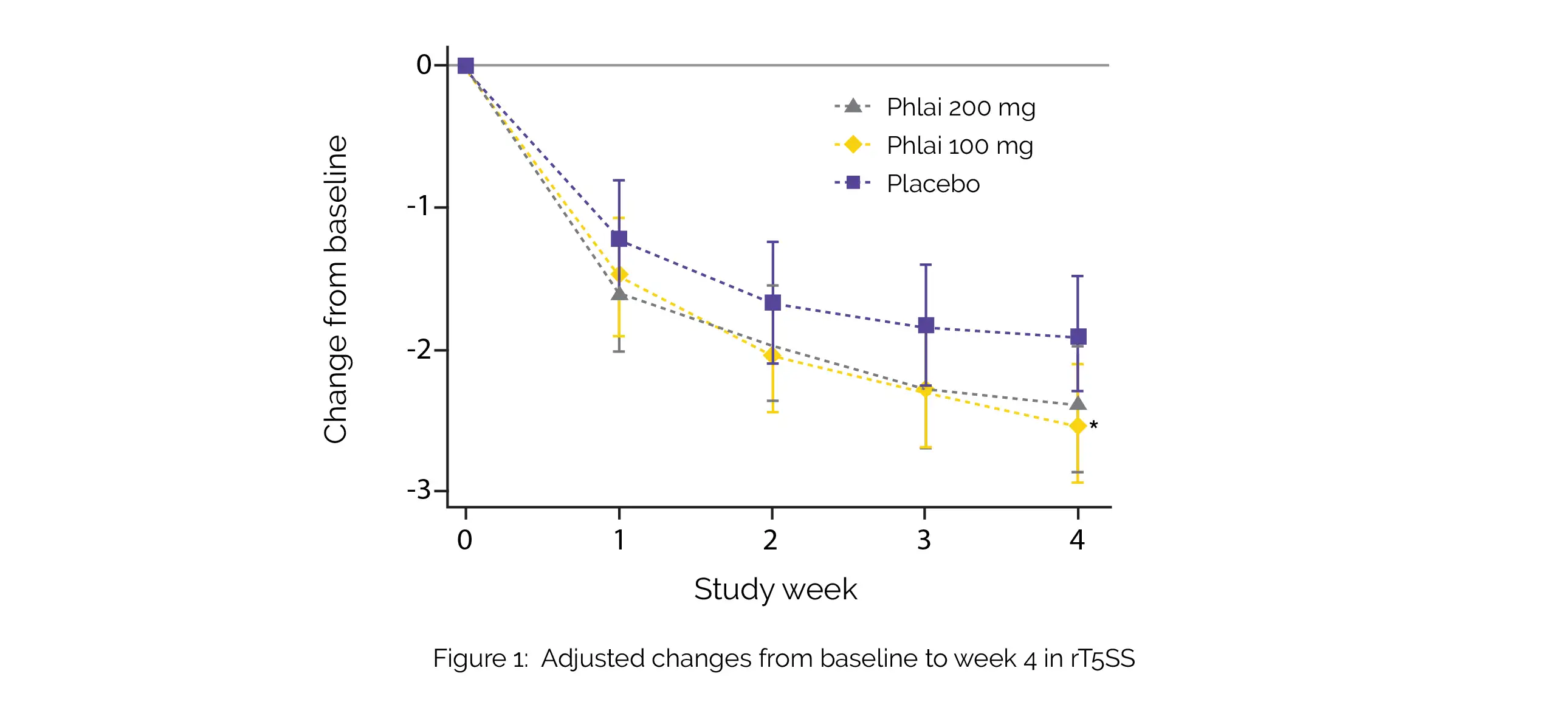
However, there was no statistical significance in PNIF, overall RCQ-36 score, iT5SS, sneezing, or nasal obstruction among the groups. Moreover, when comparing Zingiber montanum 200 mg to the 100 mg dose, no additional advantages were observed. Notably, the incidence of adverse events was comparable across all treatment groups. Phlai capsules demonstrated a good safety profile and there were modest enhancements in the rT5SS, as well as itchy eyes, itchy nose, and rhinorrhea.
These positive results pave the way for further research and larger-scale trials to better understand the effectiveness and long-term safety of Phlai capsules in managing allergic rhinitis. If future studies continue to support these findings, Phlai could become a valuable addition to the arsenal of treatments available for allergic rhinitis sufferers, providing much-needed relief from their symptoms.
Asian Pacific Journal of Allergy and Immunology
Phase III study of Phlai capsules in the treatment of allergic rhinitis: A randomized, double-blind, placebo-controlled trial
Minh P Hoang et al.
Comments (0)