Categories
Change Password!
Reset Password!


In comparison to Etoricoxib alone, a fixed-dose combination of Etoricoxib and Pregabalin provides remarkable benefits in minimizing chronic low back pain and improving functional status.
The fixed-dose combination (FDC) of 75 mg low-dose Pregabalin extended-release and 60 mg Etoricoxib showed clinically and statistically significant advantages in enhancing the functional status and lowering pain contrasted to Etoricoxib in chronic low back pain (CLBP) people, according to a phase 3, randomized study. Researchers sought to assess the safety and effectiveness of FDC of Pregabalin and Etoricoxib to treat both nociceptive and neuropathic pain components.
In adult patients with CLBP, the efficacy of FDC was compared to Etoricoxib alone on the basis of mean alteration in the numeric rating scale (NRS), rescue medication consumption, clinical global impression of improvement (CGI-I), patient global impression of improvement (PGI-I), visual analogue scale (VAS), and Roland-Morris disability questionnaire (RDQ).
Furthermore, an evaluation of safety was done. The course of intervention was 8 weeks. Overall, 319 of the 371 screened volunteers were randomized. Both treatment groups' baseline illness features and demographics didn't substantially differ from one another. At the beginning of week 4, FDC produced noticeably superior results than Etoricoxib.
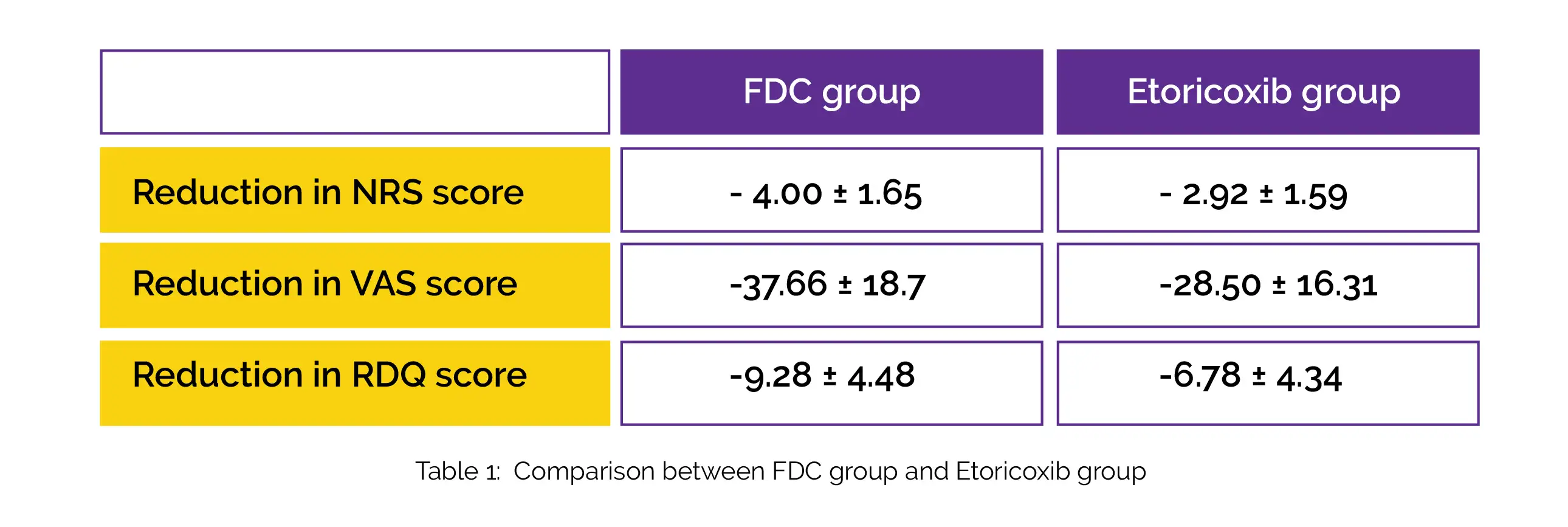
At week 8, the mean NRS score decreased in both groups from the baseline, with the mean NRS score in the FDC group being substantially lower than that of the Etoricoxib group (3.26 ± 1.56 vs 4.31 ± 1.56). At week 8, substantial reductions in VAS score and RDQ score were noted in the FDC group when compared to the Etoricoxib group, as shown in Table 1:
Additionally, the FDC group had a higher percentage of patients (36.9% vs. 5.0%) reporting their condition as ‘very much better’and doctors (36.3% vs. 5.7%) who reported that their patients' conditions had greatly improved. Overall, the study drugs were well-tolerated. When compared to Etoricoxib alone, the FDC of Etoricoxib and Pregabalin significantly improved functional status and reduced pain in individuals with CLBP.
As Pregabalin extended release-Etoricoxib FDC treats both the nociceptive and neuropathic pain components, it appears to be one of the promising therapeutic choices for CLBP, providing long-lasting and early pain relief as well as an enhancement in quality of life.
Pain and Therapy
Efficacy and Safety of Pregabalin Prolonged Release–Etoricoxib Combination Compared to Etoricoxib for Chronic Low Back Pain: Phase 3, Randomized Study
Amit B. Yeole et al.
Comments (0)