Categories
Change Password!
Reset Password!


Perioperative use of Duloxetine minimizes pain after osteotomy and also lessens the need for NSAIDs and other analgesic agents.
In people undergoing an opening-wedge high tibial osteotomy, perioperative administration of Duloxetine from two weeks prior to surgery to two weeks following surgery is beneficial for perioperative pain management and Knee Injury and Osteoarthritis Outcome Score (KOOS) improvement, as deciphered from a prospective clinical trial. The ability to manage postoperative pain is crucial for patient satisfaction.
But, no studies have examined how preoperative Duloxetine treatment affects postoperative pain after knee surgery. Thus, the purpose of this study was to ascertain whether post-high tibial osteotomy pain is decreased by perioperative Duloxetine administration. Adults (aged ≥18 years) undergoing high tibial osteotomy for medial knee osteoarthritis were incorporated in the study.
In total, 35 subjects received 40 mg/day Duloxetine and 33 subjects did not receive Duloxetine (control). The frequency of use of analgesics as well as patient-reported outcomes, such as numeric rating scale (NRS) score at rest and KOOS, were examined between the groups. With the aid of NRS scores, the quality of recovery and knee pain were assessed. On postoperative day 1, day 7, and day 14, the NRS scores of Duloxetine group were substantially lower than the control group, as shown in Table 1:
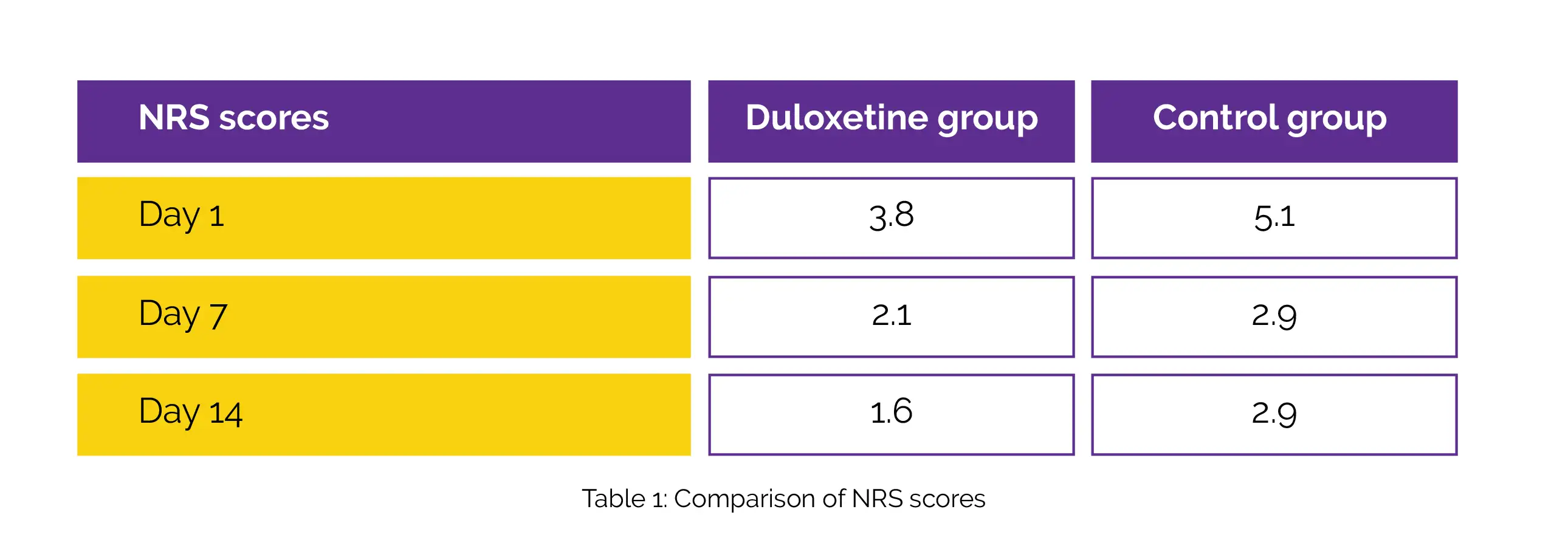
In the Duloxetine group, compared to the control group, the use of non-steroidal anti-inflammatory drug (NSAID) was considerably reduced. When compared to control arm, the Function in Sport subcategory of KOOS was remarkably improved in Duloxetine arm at 3 months after surgery, even though KOOS score was not considerably distinct in numerous subcategories at postoperative and preoperative time-points. Hence, Duloxetine is a promising agent for pain management after knee surgery.
The Knee
Perioperative Duloxetine administration reduces pain after high tibial osteotomy and non-steroidal anti-inflammatory administration: A prospective, controlled study
Shuhei Otsuki et al.
Comments (0)