Categories
Change Password!
Reset Password!


Pegozafermin demonstrates substantial improvements in fibrosis and NASH resolution across various dosage groups.
A recently published phase 2b trial in ‘The New England Journal of Medicine’ has revealed encouraging outcomes for the novel therapeutic candidate, Pegozafermin, in the treatment of nonalcoholic steatohepatitis (NASH). Rohit Loomba et al. explored the efficacy and safety of this innovative analog of glycopegylated fibroblast growth factor 21 (FGF21).
This 24-week, double-blind, multicenter trial involved the random assignment of patients with biopsy-confirmed NASH and stage F2 or F3 fibrosis (moderate or severe) to receive various doses of Pegozafermin or a placebo. The primary endpoints included fibrosis improvement, calculated by a reduction of at least one stage on a scale from 0 to 4, and resolution of NASH without worsening of fibrosis at the 24-week mark. Safety assessments were also integral to the study.
Results from the trial underscore the potential of Pegozafermin. Out of the 219 participants who received either Pegozafermin or a placebo, remarkable improvements were observed. Patients in the placebo group showed a modest 7% meeting the criteria for fibrosis improvement, while the 15-mg Pegozafermin group exhibited a substantial 22%. Even more promisingly, the 30-mg Pegozafermin group showcased a 26% improvement, and the 44-mg Pegozafermin group demonstrated a notable 27% improvement.
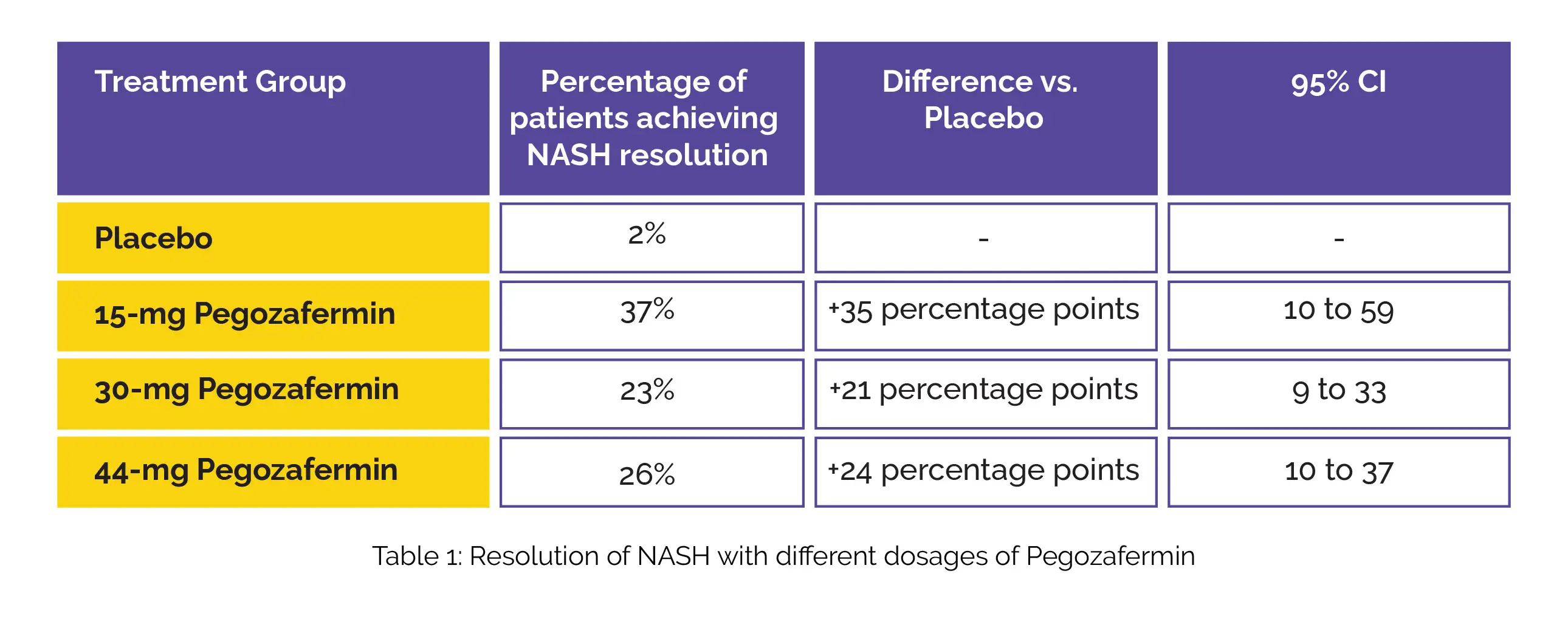
Furthermore, the study evaluated the percentage of patients achieving NASH resolution without worsening of fibrosis, showcasing a significant step forward in therapeutic efficacy as shown in Table 1:
The most commonly reported adverse events associated with Subcutaneous Pegozafermin therapy were nausea and diarrhoea. Conclusively, this phase 2b trial heralds promising strides in NASH treatment through the utilization of Pegozafermin. The improvements in fibrosis observed in the study provide robust support for advancing Pegozafermin into phase 3 development, marking a potential breakthrough in the realm of NASH therapies. As the medical community eagerly anticipates further advancements, these findings contribute significantly to the growing body of knowledge in the field of hepatology.
The New England Journal of Medicine
Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH
Rohit Loomba et al.
Comments (0)