Categories
Change Password!
Reset Password!


Orimilast is effective and safe for moderate-to-severe psoriasis management.
In a 16-week, phase 2b, dose-finding, placebo-controlled, randomized, double-blinded trial, Orismilast, an effective oral inhibitor of phosphodiesterase-4 (PDE4) B/D, exhibited a notable decrease in Psoriasis Area and Severity Index (PASI) in people with moderate-to-severe psoriasis. Furthermore, it was safe and well-tolerable consistent with the anticipated effects of PDE4 inhibition.
Richard B. Warren et al. conducted the study with the objective of assessing the effectiveness and safety of the modified-release formulation of Orismilast in adults with moderate-to-severe plaque psoriasis. Multiple imputation was employed for the analysis of efficacy outcomes. The major effectiveness outcome was the percentage alteration in PASI from study initiation to week 16.
The key secondary effectiveness outcomes included achieving a 75% decline in PASI (PASI75), attaining a skin clarity score of clear (0) or almost clear (1), and achieving a >2-point betterment in Investigator's Global Assessment (IGA 0/1) at week 16. Out of the 202 patients randomized, baseline characteristics were comparable between the groups, except for a higher proportion of severe disease in the Orismilast group compared to the placebo group.
Orismilast demonstrated substantial enhancements in the major outcome, the percentage alteration in PASI, from baseline to week 16 (Orismilast –52.6% to –63.7%, and placebo –17.3%; all). A higher percentage of individuals receiving Orismilast achieved PASI75 (39.5%-49.0%) and PASI90 (22.0%-28.3%; for 20 and 40 mg) compared to placebo (PASI75, 16.5%, and PASI90, 8.3%) at week 16, as depicted in Figure 1:
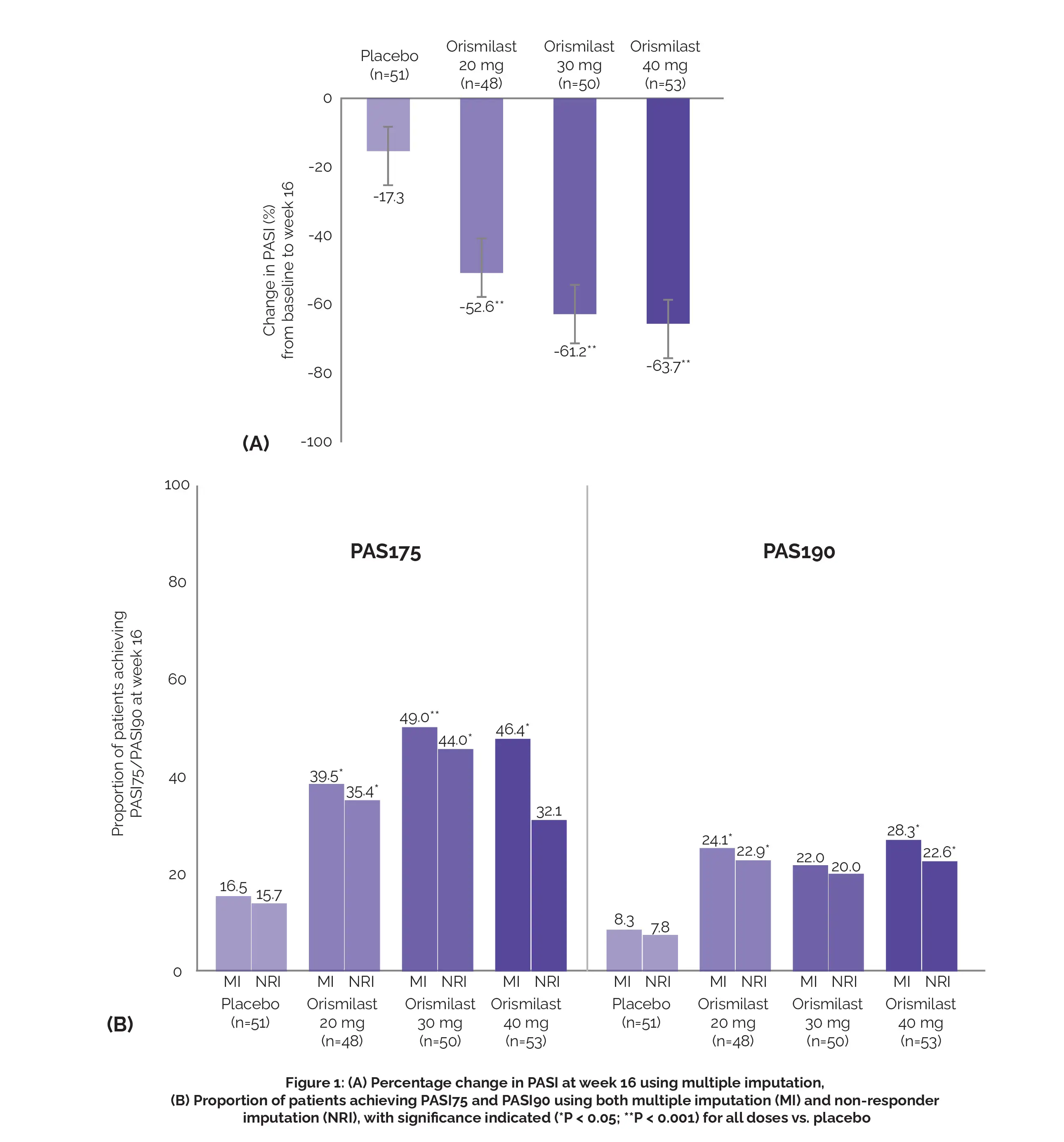
The safety results were consistent with the anticipated outcomes for PDE4 inhibition, and there were observable tolerability effects dependent on the dosage. Thus, Orismilast exhibited superior efficacy compared to the placebo, accompanied by a safety profile consistent with the effects of PDE4 inhibition.
Journal of the American Academy of Dermatology
Orismilast in moderate-to-severe psoriasis: Efficacy and safety from a 16-week, randomized, double-blinded, placebo-controlled, dose-finding, phase 2b trial (IASOS)
Richard B. Warren et al.
Comments (0)