Categories
Change Password!
Reset Password!


Once-daily Roflumilast cream markedly improves the signs and symptoms of atopic dermatitis in patients aged ≥ 6 years, with a favorable safety profile.
Roflumilast cream 0.15%, a potent phosphodiesterase 4 inhibitor, has emerged as a promising non-steroidal, once-daily treatment for moderate-to-severe atopic dermatitis (AD) in two Phase 3 trials (INTEGUMENT-1 and INTEGUMENT-2) led by E. Simpson and colleagues. Conducted on over 1,300 patients (Eczema Area and Severity Index [EASI] ≥5) aged 6 years and older, the trials demonstrated substantial improvements in skin condition, patient-reported outcomes, and family impact. The goal was to explore the cream's effectiveness, safety, and tolerability for AD management.
Participants received either 0.15% Roflumilast cream or a vehicle for 4 weeks. The primary outcome measure was validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) success at week 4, defined as clear/almost clear skin with at least a 2-grade betterment. Secondary outcomes encompassed changes in the Dermatitis Family Impact (DFI), SCORing atopic dermatitis (SCORAD) score, and Patient-Oriented Eczema Measure (POEM). Safety and tolerability were examined by assessing treatment-emergent adverse events (TEAEs) and cessations.
Among 884 and 453 participants who received Roflumilast and vehicle, respectively, a markedly higher percentage of Roflumilast-treated patients attained vIGA-AD success at week 4. Improvements (least squares mean differences) in DFI, SCORAD total score, and POEM were more pronounced with Roflumilast. Both treatments were associated with low rates of TEAEs and TEAEs leading to discontinuation (Table 1).
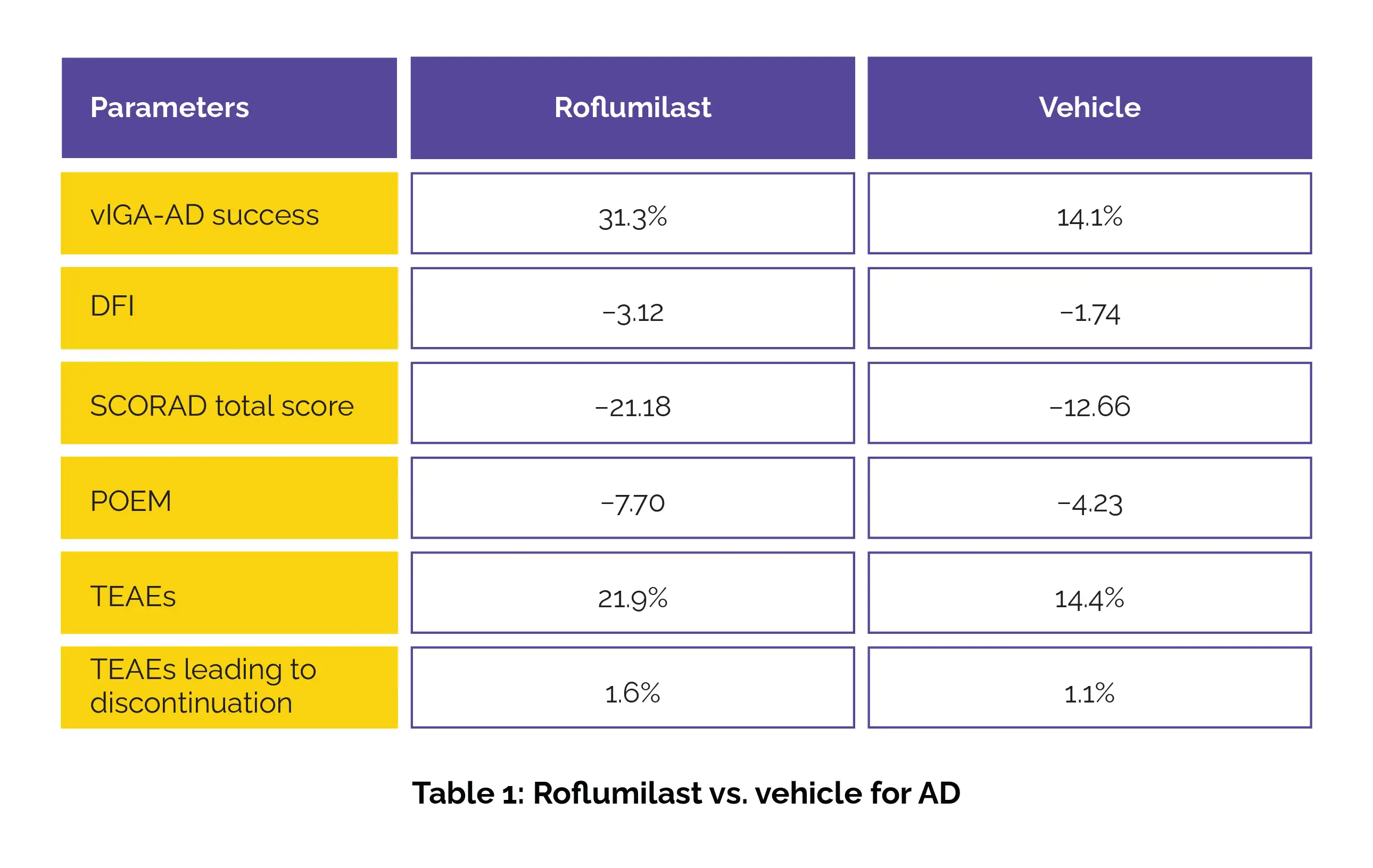
These findings underline the potential of once-daily 0.15% Roflumilast cream as a safe and effective therapy for tackling mild to moderate AD in patients aged 6 and above.
Annals of Allergy
Patient-reported outcomes and family impact with Roflumilast cream in atopic dermatitis: pooled phase 3 results
E. Simpson et al.
Comments (0)