Categories
Change Password!
Reset Password!


In healthy adults, heterologous prime-boost vaccination with 1 dose of NVSI-06-08 after 2 doses of BBIBP-CorV was immunogenic, tolerant, and safe.
A randomized, double-blind, controlled, phase 2 trial depicted that NVSI-06-08, a broad-spectrum synthetic COVID-19 vaccine, is effective and safe when given after two doses of BBIBP-CorV. Investigators aimed to assess NVSI-06-08 safety and immunogenicity as a heterologous booster vaccination in BBIBP-CorV receivers.
Healthy people aged 18 years or more were divided into three groups (600 participants in each group). Participants who received two doses of BBIBP-CorV 4-6, 7-9, and more than 9 months earlier were randomized 1:1 to get either NVSI-06-08 (heterologous booster) or BBIBP-CorV (homologous booster). The incidence of noxious effects was low and the overall safety profile was fairly identical between 2 booster regimens.
Both IgG antibodies and neutralizing antibodies triggered by the NVSI-06-08 booster were greater than those from the BBIBP-CorV booster against not only the SARS-CoV-2 prototype strain but also other variants of concern. As shown in Table 1, the geometric mean titer (GMT) against the Omicron variant induced by heterologous NVSI-06-08 booster was substantially greater than that induced by BBIBP-CorV.
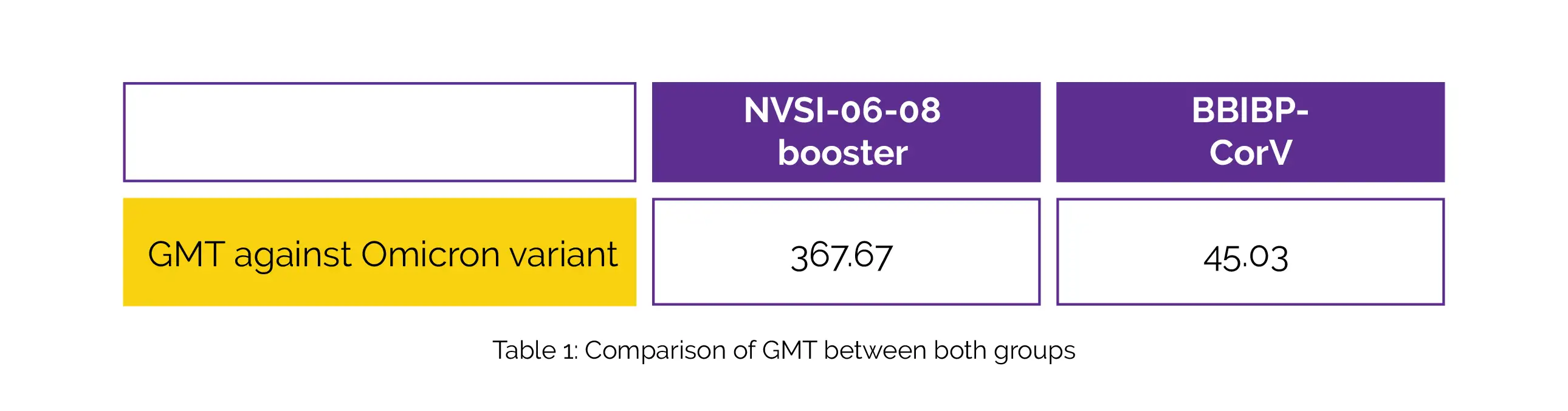
NVSI-06-08 appears to be a safe and immunogenic booster dosage after 2 BBIBP-CorV doses and shows immunological superiority to another BBIBP-CorV homologous boost dose.
Nature Communications
Safety and immunogenicity of a hybrid-type vaccine booster in BBIBP-CorV recipients in a randomized phase 2 trial
Nawal Al Kaabi et al.
Comments (0)