Categories
Change Password!
Reset Password!


In people suffering from oral lichen planus, a new mucoadhesive clobetasol patch is safe and effectively improves patient-reported outcomes.
In individuals diagnosed with erosive oral lichen planus and measurable symptomatic ulcers, a novel mucoadhesive clobetasol patch exhibited a favorable safety profile and superiority to placebo in terms of improvement in pain, ulcer area, symptom severity, disease activity, and quality of life, according to a phase 2 randomized clinical trial published in Journal of Oral Pathology & Medicine. This study reported the safety and efficacy of the new clobetasol patch to treat oral lichen planus (a chronic inflammatory disorder of the oral mucosa).
In this double-blind, placebo-controlled, multicenter study, 138 participants (99 females and 39 males) were randomly allocated to get clobetasol patch (1, 5, 20 µg) or placebo (identical nonmedicated patch) twice daily for four weeks. Alteration in total ulcer area compared to baseline was the major outcome ascertained while an improvement from baseline in pain, quality of life, and disease activity were the secondary outcomes ascertained.
Data were examined and expressed as mean [standard deviation]. According to the findings of the statistical analyses, treatment with 20-μg clobetasol patch displayed remarkable improvements in ulcer area, symptom severity, disease activity, pain, and quality of life when compared to placebo.
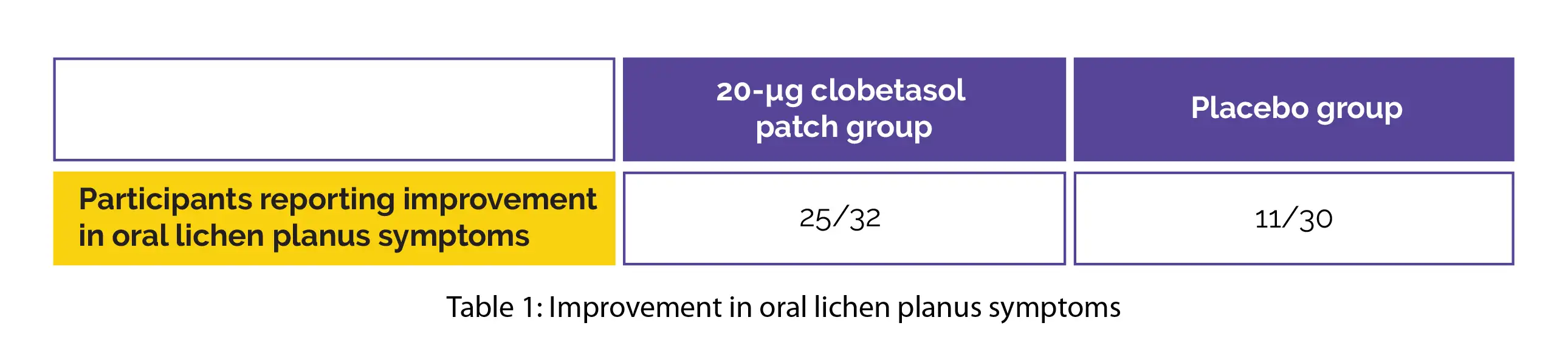
In comparison with the placebo group, the 20-µg clobetasol patch group exhibited remarkable improvements in oral lichen planus symptoms (very much better [best rating]) from beginning to the end of the trial, as shown in Table 1:
Only mild/moderate adverse events were reported. The occurrence of candidiasis was low (2%).
Thus, the novel mucoadhesive clobetasol patch is effective and safe for the management of oral lichen planus.
Journal of Oral Pathology & Medicine
Efficacy and safety of a novel mucoadhesive clobetasol patch for treatment of erosive oral lichen planus: A phase 2 randomized clinical trial
Michael T Brennan et al.
Comments (0)