Categories
Change Password!
Reset Password!


Dual therapy with Vonoprazan - Amoxicillin can be a suitable alternative to conventional therapy, with effective and safe H. pylori eradication, even in cases of clarithromycin resistance.
Xiaolei Wang and colleagues have unveiled a significant advancement in the treatment of Helicobacter pylori (H. pylori) infection, a condition increasingly challenging due to the surge in antibiotic resistance as mentioned in the leading journal ‘Therapeutic Advances in Gastroenterology'. With antibiotic resistance on the rise, conventional H. pylori eradication therapies have seen a decline in their effectiveness.
In response to this pressing issue, a potential solution has emerged in the form of Vonoprazan–Amoxicillin (VA) dual therapy. This rigorous single-centre, open-label, randomized controlled trial aimed to determine the safety and effectiveness of VA dual therapy with a standard Bismuth quadruple therapy containing Amoxicillin and Clarithromycin for management of H. pylori infection. Treatment-naïve patients with H. pylori infection were randomly assigned in a 1:1 ratio to either:
Utilizing real-time polymerase chain reaction (PCR), the study also assessed Clarithromycin resistance and CYP2C19 gene polymorphisms. Researchers analyzed eradication rates and adverse events. The study enrolled 151 patients and yielded promising outcomes. The eradication rates in the VA group, assessed through intention-to-treat (ITT), modified ITT (mITT), and per-protocol (PP) analyses, demonstrated noninferiority than RBAC group, as shown in Table 1:
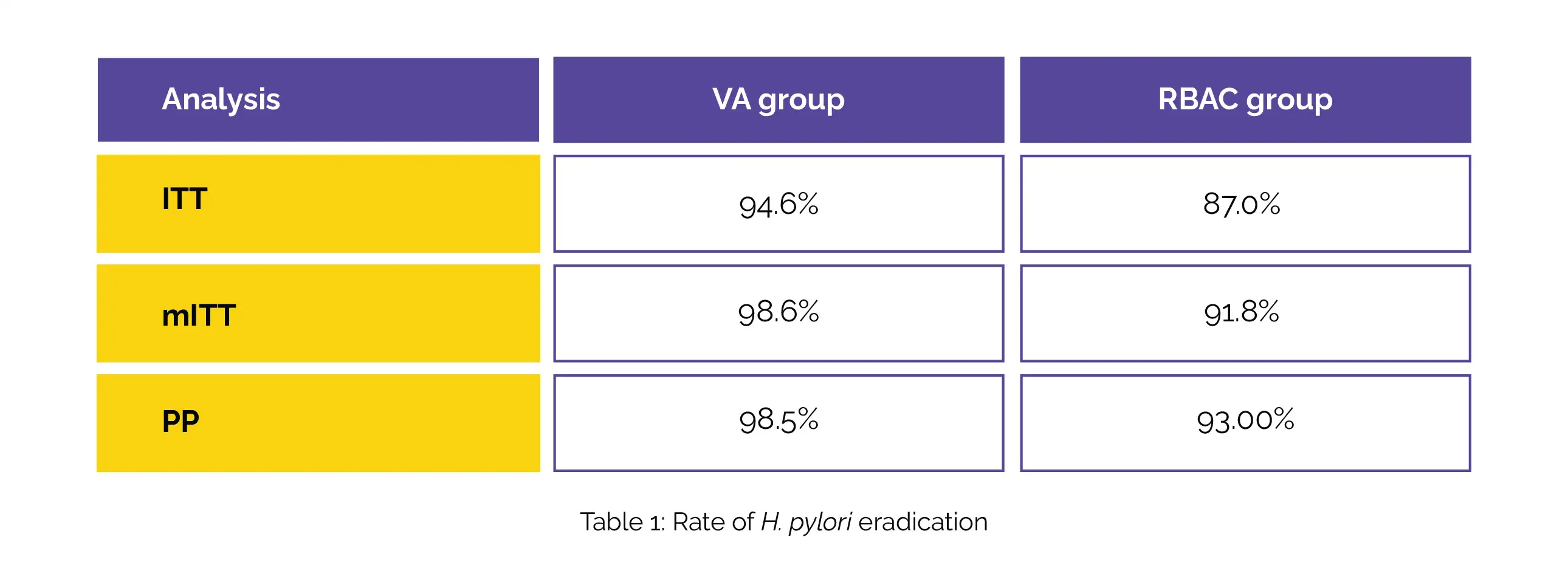
Particularly noteworthy was the observation that in patients with Clarithromycin-resistant strains, the RBAC group's eradication rate fell below 90%, although it did not significantly differ from the VA group. Another notable finding was the lower incidence of adverse events in the VA group at 39.2%, which was markedly lower than the 79.2% seen in the RBAC group.
With fewer adverse reactions, VA dual therapy exhibited noninferior effectiveness to RBAC in first-line H. pylori elimination. This research marks an impending paradigm shift in H. pylori therapy, proposing renewed hope for patients with lessened side effects. The implications extend beyond the study, potentially benefitting individuals grappling with antibiotic resistance in eradication therapy.
Therapeutic Advances in Gastroenterology
Efficacy and safety of vonoprazan–amoxicillin dual therapy for Helicobacter pylori first-line treatment: a single-center, randomized, controlled trial
Xiaolei Wang et al.
Comments (0)