Categories
Change Password!
Reset Password!


Remibrutinib was safe and effective to treat chronic spontaneous urticaria.
Patients with chronic spontaneous urticaria treated with Remibrutinib, a new Bruton’s tyrosine kinase (BTK) inhibitor, had reduced disease activity and sustained improvements throughout the treatment. Moreover, it illustrated a favorable safety profile across the entire dose range in a randomized, dose-finding, placebo-controlled, Phase 2b, double-blind study. Researchers sought to describe the dose-response and assess the safety and effectiveness of Remibrutinib in urticaria management.
Urticaria patients that were not adequately managed by second-generation H1-antihistamines, whether they previously had anti-immunoglobulin E therapy or not, were included. Remibrutinib was administered to patients at doses of 10, 35, or 100 mg once day (q.d.), 10 mg two times a day (b.i.d.), 25 mg twice daily (b.i.d.), or a placebo (1:1:1:1:1:1:1 ratio). The primary outcomes ascertained were safety and weekly alteration in baseline Urticaria Activity Score (UAS7) from Week 4.
A total of 311 subjects were randomized. All Remibrutinib dosages were associated with decreased symptom scores from Week 1 to Week 12, with UAS7 alteration from baseline at Week 4 being -19.1 (10 mg q.d.), -19.1 (35 mg q.d.), -14.7 (100 mg q.d.), -16.0 (10mg b.i.d.), -20.0 (25 mg b.i.d.), -18.1 (100 mg b.i.d.), and -5.4 for placebo, as shown in Figure 1:
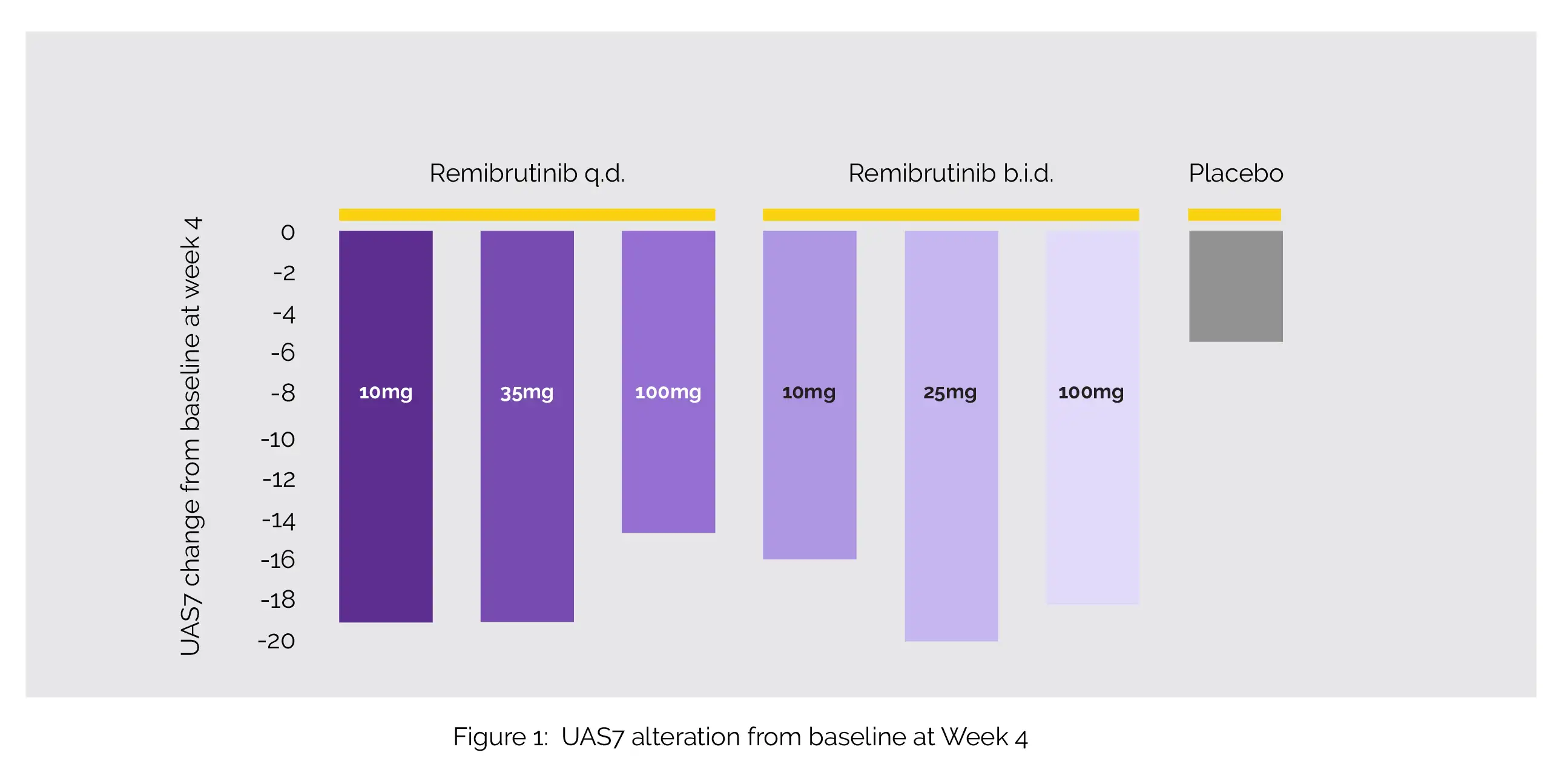
The majority of side effects lacked a dose-dependent pattern and were mild to moderate. With a quick onset of action and a promising safety profile, Remibrutinib was successful in managing chronic spontaneous urticaria across the whole dosage range.
Journal of Allergy and Clinical Immunology
Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria
Marcus Maurer et al.
Comments (0)