Categories
Change Password!
Reset Password!


In migraine patients, non-invasive vagus nerve stimulation is effective and safe to reduce monthly migraine days.
The findings of a recent study suggested the clinical utility of non-invasive vagus nerve stimulation to prevent migraine, especially for adults who have migraine with aura, and also reinforced the well-established tolerability and safety profile of this therapy. Researchers undertook this randomized, sham-controlled, double-blind, PREMIUM II trial to determine safety and efficacy of non-invasive vagus nerve stimulation to prevent migraine.
Following completion of a four-week diary run-in period, migraine patients with or without aura were randomized to get active non-invasive vagus nerve stimulation or sham treatment during a twelve-week double-blind period. Out of 336 recruited people, 113 (active, n = 56; sham, n = 57) finished ≥70 days of double-blind period and were ≥66% adherent with therapy, encompassing the prespecified modified intention-to-treat population.
The coronavirus pandemic resulted in the early termination of the study. Compared to the sham group, the active group exhibited a higher mean decline in monthly migraine days (major outcome) and greater responder rate (i.e. the percentage of people with a ≥50% decline in migraine days), as shown in Table 1:
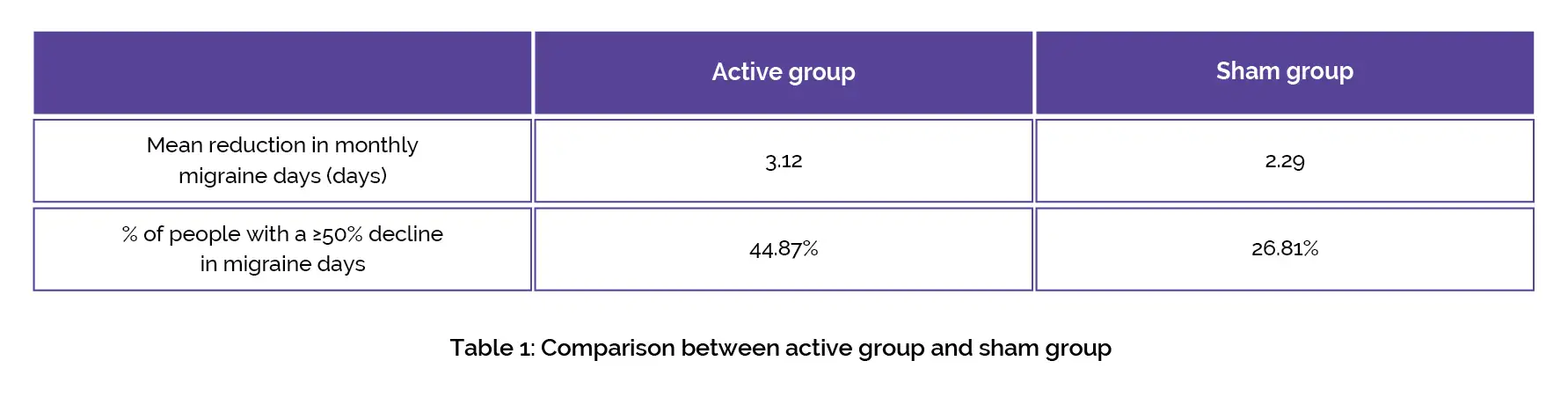
The prespecified subgroup assessment indicated that people with aura responded preferentially. No severe device-associated side effects were noted. Thus, non-invasive vagus nerve stimulation is beneficial for migraine prevention.
Cephalalgia
Non-invasive vagus nerve stimulation for prevention of migraine: The multicenter, randomized, double-blind, sham-controlled PREMIUM II trial
Umer Najib et al.
Comments (0)