Categories
Change Password!
Reset Password!


Nirsevimab proved effective in averting respiratory syncytial virus-related hospitalizations in infants (aged <12 months).
A study featured in “The New England Journal of Medicine” disclosed that Nirsevimab (a monoclonal antibody) provided protection against hospitalization for respiratory syncytial virus (RSV)-associated lower respiratory tract infection, including very severe cases, in conditions closely resembling real-world settings for infants.
This study led by Simon B. Drysdale and his team explored the safety of Nirsevimab and its effect on hospitalizations for RSV-associated lower respiratory tract infection (RSV-LRTI) when used in healthy infants. Overall, 8058 infants aged 12 months or younger (born at gestational age = minimum 29 weeks), and entering their initial RSV season in Western European countries were allocated in a 1:1 ratio at random. They were either administered with Nirsevimab intramuscular injection (number of infants=4037) or underwent standard care (no intervention) (number of infants=4021) prior to or during the RSV outbreaks.
The primary endpoint was hospitalization due to RSV-LRTI, characterized by both admissions to the hospital and a positive result indicating RSV infection. Acute RSV-LRTI, defined as hospitalization for RSV-LRTI with an oxygen saturation of <90% and the need for supplemental oxygen was considered as the secondary endpoint. The details of Nirsevimab efficacy have been described below (Table 1):
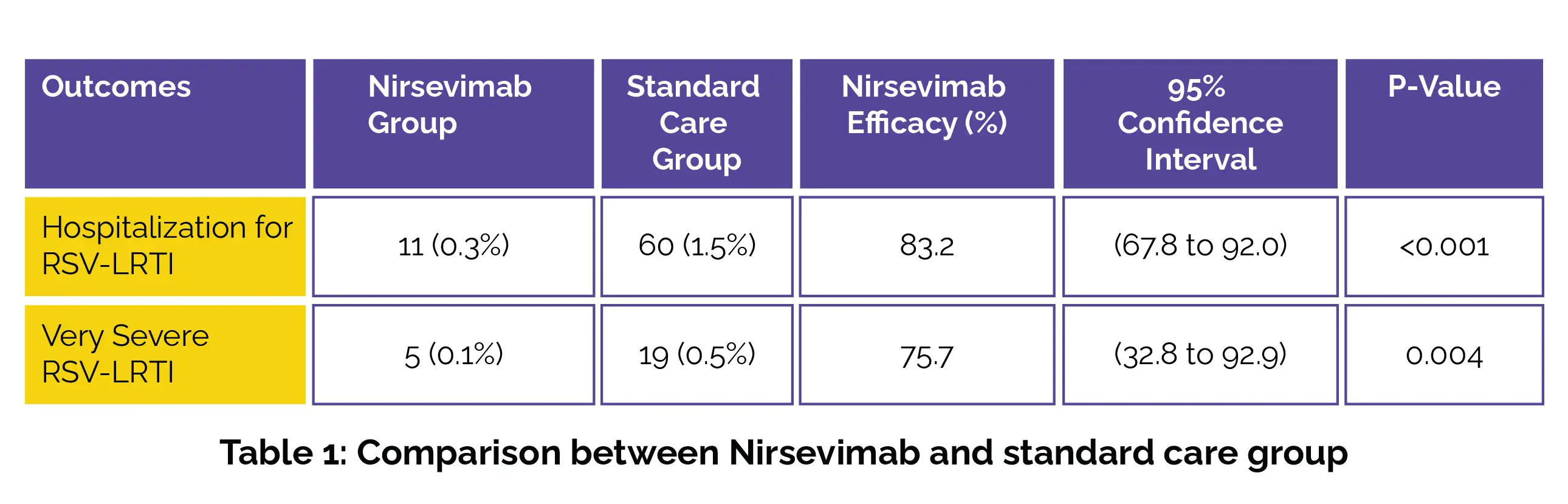
The effectiveness of Nirsevimab against hospitalization for RSV-associated LRTI was 89.6% in France, 74.2% in Germany and 83.4% in the UK. Eighty-six infants (2.1%) in the Nirsevimab group reported therapy-linked adverse events.
The New England Journal of Medicine
Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants
Simon B Drysdale et al.
Comments (0)