Categories
Change Password!
Reset Password!


FDA-approved Linaclotide enhances bowel movement frequency and stool consistency, making it an effective therapy for treating constipation in children.
The outcomes of a study found Linaclotide to be well-tolerated and effective for functional constipation in children, as published in The Lancet Gastroenterology & Hepatology.
Carlo Di Lorenzo performed a randomised, double-blind, placebo-controlled, phase 3 study at 64 hospital sites in 7 countries (USA, Canada, Israel, Italy, the Netherlands, Ukraine, Estonia). This study aimed to evaluate both the safety and effectiveness of linaclotide (guanylate cyclase-C agonist) in children suffering from functional constipation.
Children aged 6-17 who fulfilled the Rome III criteria for functional constipation were randomly given either linaclotide 72 μg orally or a placebo once per day for 12 weeks. The change from baseline (CFB) in spontaneous bowel movements (SBMs) per week and CFB in stool consistency (firmness) over the 12 weeks were considered as the primary and secondary endpoints, respectively. The analysis included all patients who received a minimum of 1 dose of the study drug for efficacy and safety assessment.
From Oct 1, 2019, to March 21, 2022, 330 children were registered and randomly divided into linaclotide (n=166) or placebo (n=164) groups. Safety and efficacy endpoints were evaluated in 328 patients (164 patients in each group) as 2 children in the linaclotide group received no therapy. Of the total, 293 children (89%) completed the specified treatment period (328 females [55%, 147 males [45%]). The mean frequency rate for SBMs at baseline and during the intervention is shown in the following Table 1:
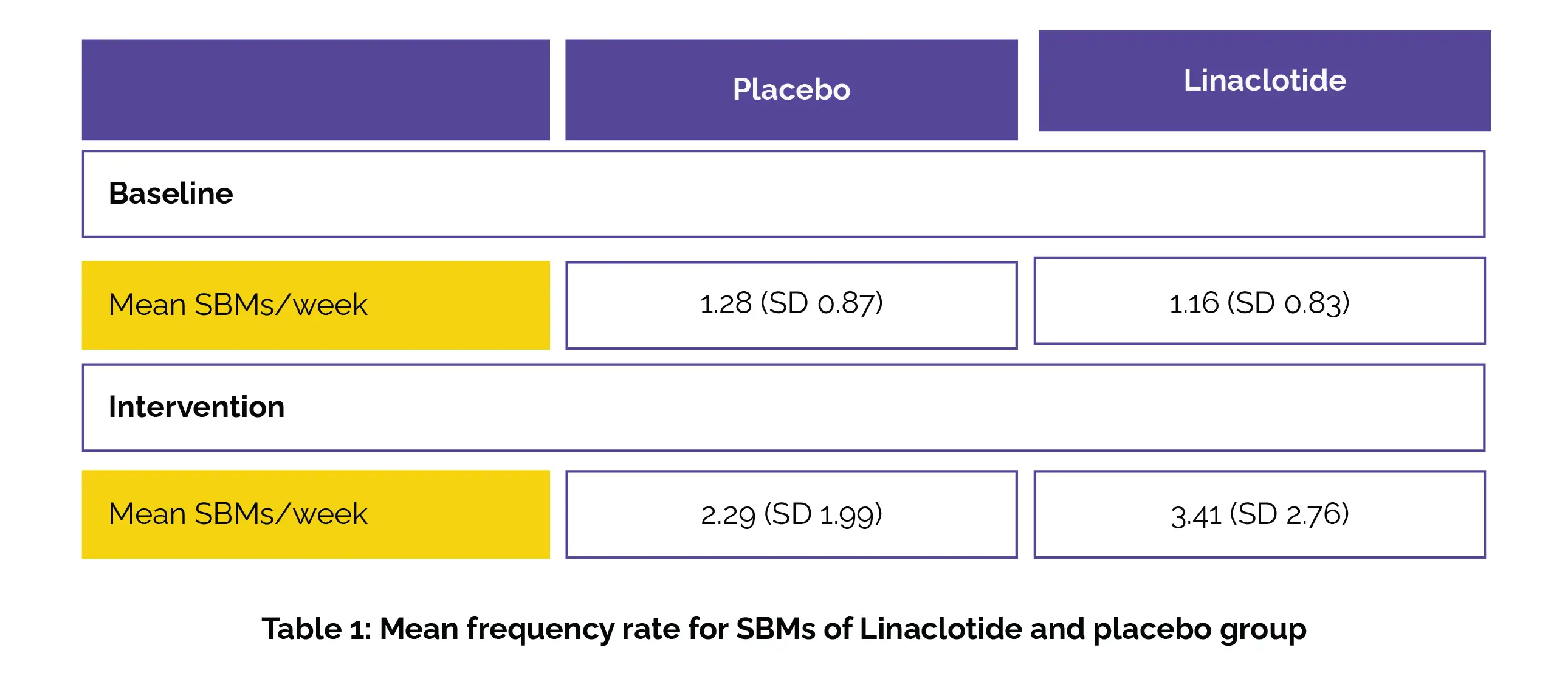
Compared with placebo, the children treated with linaclotide showed significant improvement in SBM frequency and stool consistency over placebo.
"During the study, patients treated with linaclotide mostly reported experiencing diarrhoea as a treatment-emergent adverse event (TEAE), whereas those on placebo predominantly reported COVID-19. The most common treatment-related TEAE was diarrhoea. Additionally, a serious adverse event involving severe dehydration-induced diarrhoea requiring hospitalization occurred in a 17-year-old female patient from the linaclotide group. Fortunately, the issue was resolved without any lasting complications after receiving intravenous (IV) fluids. No fatalities were recorded.
The Lancet Gastroenterology & Hepatology
Efficacy and safety of linaclotide in treating functional constipation in paediatric patients: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial
Carlo Di Lorenzo et al.
Comments (0)