Categories
Change Password!
Reset Password!


Keverprazan was non-inferior to Lansoprazole to heal duodenal ulcers.
According to a phase II randomized, double-blind, parallel-controlled trial published in the "Journal of Gastroenterology and Hepatology", Keverprazan, a novel potassium-competitive acid blocker was non-inferior to Lansoprazole in the treatment of duodenal ulcers. Researchers aimed to explore the effectiveness, safety, and dose-effect relationship of Keverprazan (20 or 30 mg) in comparison to Lansoprazole (30 mg one time a day for 4 to 6 weeks) in the treatment of duodenal ulcer confirmed by endoscopy.
Overall, 168 people (93.33%) of the 180 participants who were randomly assigned-55 cases in the Keverprazan 20 mg group, 61 cases in the Keverprazan 30 mg group, and 64 cases in the Lansoprazole 30 mg group-completed the research. The proportions of cured duodenal ulcer participants in the Keverprazan 20 mg, Keverprazan 30 mg, and Lansoprazole 30 mg groups at week 4 and week 6, are shown in Table 1:
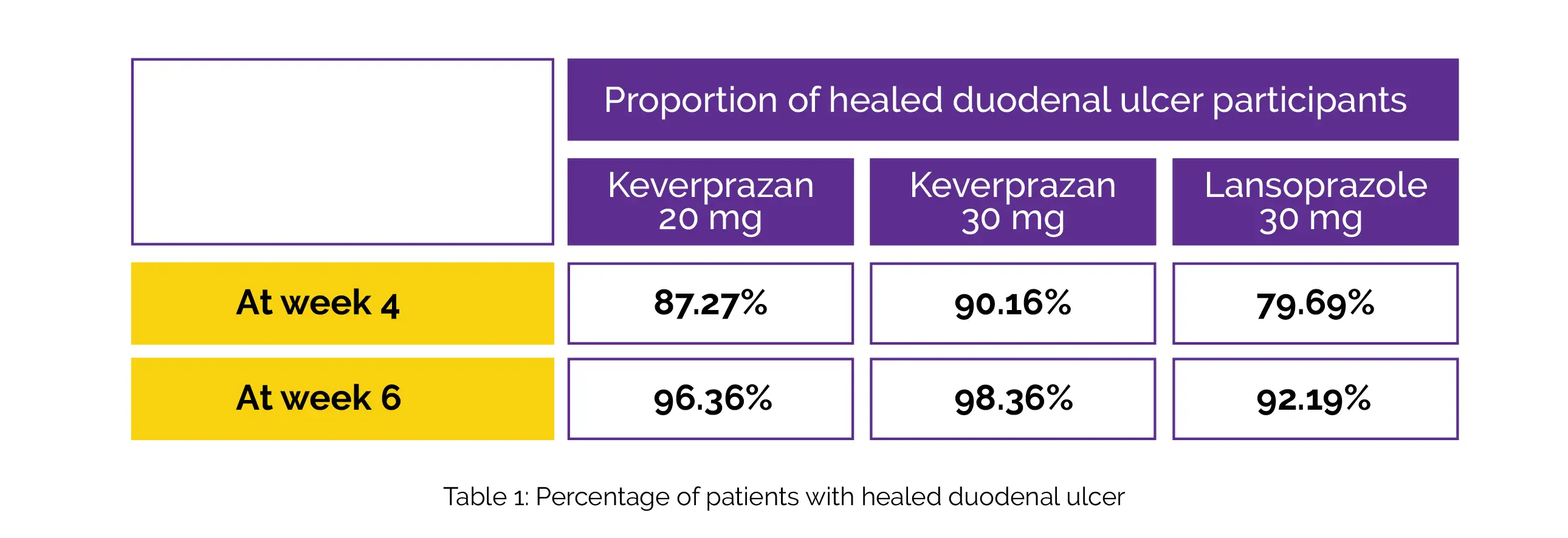
When compared to the groups receiving Lansoprazole 30 mg and Keverprazan 30 mg, the incidence of adverse events was reduced in the Keverprazan 20 mg group. Keverprazan was shown to be efficacious and non-inferior to Lansoprazole to treat duodenal ulcers. Based on comparable safety and effectiveness data, 20 mg of Keverprazan once a day is suggested for the follow-up research of acid-linked diseases.
Journal of Gastroenterology and Hepatology
Efficacy of Keverprazan for duodenal ulcer: A phase II randomized, double-blind, parallel-controlled trial
Nian-di Tan et al.
Comments (0)