Categories
Change Password!
Reset Password!


According to a randomized controlled clinical study, both bismuth-containing quadruple therapy and high-dose dual therapy were effective, safe, and had high compliance in H. pylori-infected patients. Researchers sought to develop a novel elimination regimen as a first-line treatment option for individuals with H. pylori infection and to assess the safety and effectiveness of high-dose Amoxicillin-proton pump inhibitor dual therapy.
Overall, 971 H. pylori-positive subjects who were given first treatment were enlisted and randomly assigned to the treatment group and the control group. For 14 days, volunteers in the treatment group were given 750 mg of Amoxicillin 4 times per day and 20 mg of Esomeprazole 4 times per day.
For 14 days, the control group was given 250 mg of Clarithromycin capsules two times a day, 220 mg of bismuth potassium citrate two times a day, 20 mg of Esomeprazole twice daily, and 1000 mg of Amoxicillin twice daily. The urea breath test was examined four weeks following the conclusion of therapy to see if H. pylori had been eliminated.
The extent of symptom remission before and after initial treatment, compliance, total clinical symptom scores prior to and post-initial therapy, gender, and age, were not statistically different between both the groups. H. pylori elimination rates in the dual therapy and quadruple therapy according to intention-to-treat (ITT) analysis, modified intention-to-treat (mITT) analysis, and per protocol (PP) analysis, is shown in Table 1:
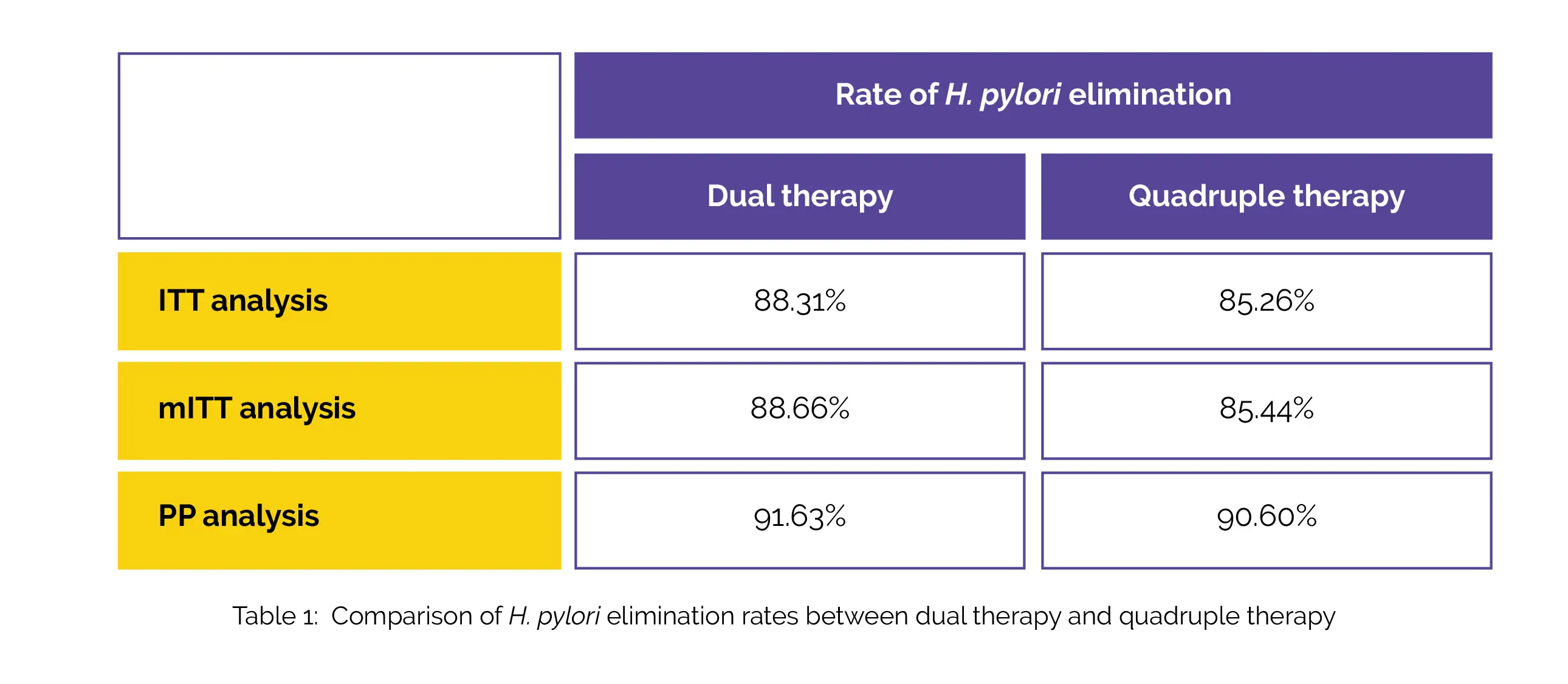
Adverse events were much fewer in the dual therapy group (13.3%) when compared to the quadruple therapy group (28.2%). As the empirical first-line therapeutic option for the eradication of H. pylori infection, both bismuth-containing quadruple regimen and high-dose dual regimen can be suggested.
Annals of Medicine
The prospective multiple-centre randomized controlled clinical study of high-dose Amoxicillin-proton pump inhibitor dual therapy for H. pylori infection in Sichuan areas
Cheng Shen et al.
Comments (0)