Categories
Change Password!
Reset Password!


Compared to intravenous route, Ketorolac administered intranasally is non-inferior to alleviate severe renal colic.
An effective, non-invasive, and fast alternative analgesia option can be provided by intranasal delivery of Ketorolac, which showed non-inferiority to intravenous administration in a double-blind, non-inferiority, randomized controlled trial. U. Al-Khalasi et al. compared the effectiveness of intranasal Ketorolac to intravenous Ketorolac in treating acute renal colic patients.
Patients who had been diagnosed clinically with severe renal colic using the Visual Analogue Scale (VAS) were included. Adult subjects (18-64 years of age) who had a VAS of 7 or above were included. People who recently used nonsteroidal anti-inflammatory drugs (NSAIDs) within 8 hours of presentation or were ineligible for NSAIDs were excluded.
Patients were randomly assigned in a 1:1 ratio to receive either 30 mg intranasal Ketorolac + intravenous normal saline or intravenous Ketorolac 30 mg + intranasal normal saline as a single dose using a computer-generated randomization sequence. A decrease in pain scores after 60 minutes was the main outcome. At the baseline (VAS 0), at the end (VAS 30), and after 60 minutes, pain scores were collected. Additionally, adverse events of therapy were noted. If upper limit of two-sided 95% confidence interval (CI) for VAS difference was < 2 points, non-inferiority was demonstrated. To examine the outcomes, intent-to-treat analyses was utilized.
Out of 171 randomized participants, 86 received Ketorolac intravenously, and 85 received it intranasally. The initial pain levels, presence of obstructive stones, stone sizes and locations, and baseline features of the patients in the two groups were comparable. When Ketorolac was administered intravenously and intranasally, the mean difference between VAS 0 and VAS 60 is depicted in Table 1:
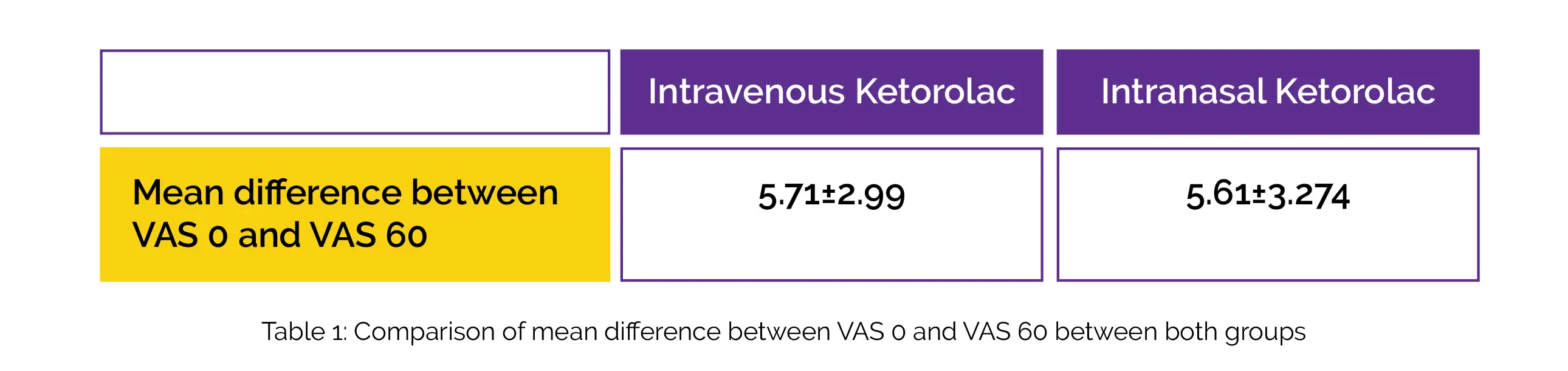
The upper limit of the 95% CI was below the non-inferiority margin, and the difference in mean pain reduction between groups after 60 minutes was 0.22 (95% CI - 0.75, 1.20). When comparing patients who were administered intravenous Ketorolac to those getting intranasal Ketorolac, 69.8% (n = 60) subjects experienced a response to therapy (defined as a VAS of 3 or below at 60 min).
Notably, 11.6% of subjects (n = 10) in the intravenous group and 10.6% of subjects (n = 9) in the intranasal group both required rescue analgesia. In this trial, no considerable adverse events were found. Thus, Ketorolac is a promising acute pain reliever for individuals with severe renal colic.
Annals of Emergency Medicine
Atomized Intranasal Ketorolac Versus Intravenous Ketorolac for Treatment of Severe Renal Colic in the Emergency Department: A Double-blind, Non-inferiority, Randomized Controlled Trial
U. Al-Khalasi et al.
Comments (0)