Categories
Change Password!
Reset Password!


A single dose of intramuscular AZD7442 is effective to prevent SARS-CoV-2 infection, without apparent safety concerns.
In a study published in The New England Journal of Medicine, AZD7442 (composed of tixagevimab and cilgavimab) was found to be beneficial for preexposure prophylaxis against coronavirus disease in adults who had an elevated risk of an unsatisfactory response to vaccination, a greater risk of exposure to coronavirus, or both. This study reported the findings from the ongoing, phase III PROVENT trial that assessed the monoclonal antibody combination AZD7442 to prevent symptomatic and severe coronavirus disease in adults.
The trial recruited participants who had an elevated risk of unsatisfactory response to vaccination against coronavirus disease, a higher risk of exposure to coronavirus, or both. A total of 5197 volunteers (≥18 years of age) were randomly allocated in a 2:1 ratio to get a single dose (2 consecutive intramuscular injections, 1 containing cilgavimab and the other containing tixagevimab) of either 300 mg of AZD7442 (n=3460) or saline placebo (n=1737).
The volunteers were followed up for upto 183 days in primary assessment. The occurrence of symptomatic coronavirus disease (RT-PCR confirmed viral infection) following treatment with AZD7442 or placebo and on or prior to day 183 was the efficacy outcome ascertained. The occurrence of adverse effects following a single dose of AZD7442 was the safety outcome ascertained.
After 30% of volunteers were aware of their randomized assignment, the primary analysis was performed. Overall, 34.2% (593/1736) in the placebo group and 35.3% (1221/3461) in the AZD7442 group reported having at least one adverse effect, majority of which were moderate or mild in severity. The incidence of symptomatic COVID-19 was less in the AZD7442 group vs. placebo group (relative risk decrease, 76.7%), as shown in Table 1:
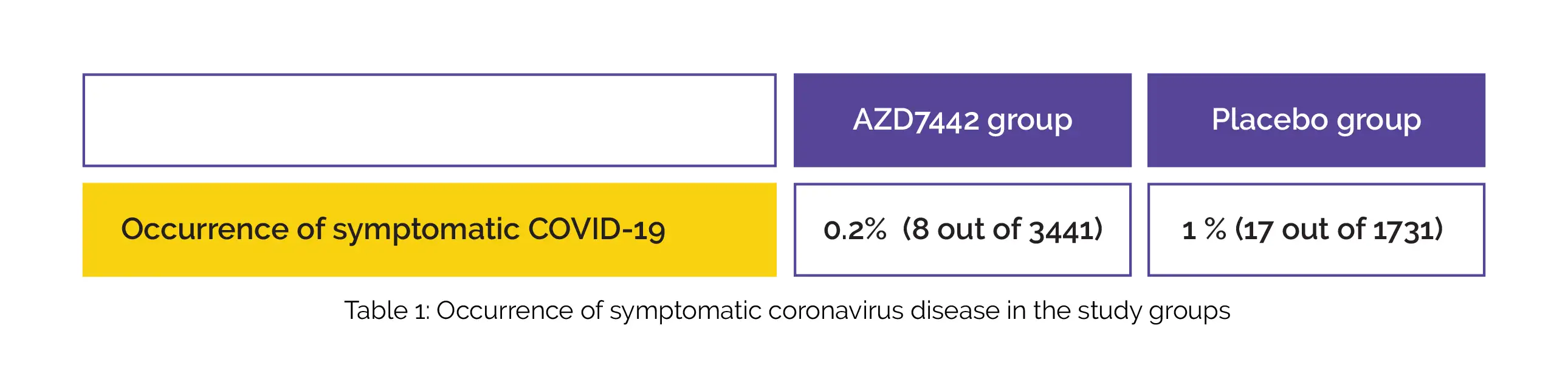
In the placebo group, 5 cases of serious or critical coronavirus disease and two deaths associated with SARS-CoV-2 occurred. Hence, the findings of this study supported the usage of AZD7442 as immunoprophylaxis for COVID-19 prevention.
The New England Journal of Medicine
Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19
Myron J. Levin et al.
Comments (0)