Categories
Change Password!
Reset Password!


Long intervals of the inactivated SARS-CoV-2 vaccine resulted in better immunogenicity rates among the high-risk occupational population.
The findings of a recent randomized controlled trial showed a higher SARS-CoV-2 neutralizing antibody response at a longer interval (21 and 28 days) compared to shorter antibody interval (14 days) among individuals who face a greater occupational risk exposure to the SARS-CoV-2 infection.
The present randomized controlled trial aimed to evaluate the safety, immunogenicity and appropriate vaccination interval of inactivated SARS-CoV-2 vaccine among high-risk occupational people. People aged 18 to 59 years were enrolled and administered with 2 doses of inactivated COVID-19 vaccine on a duration of 14, 21, and 28 days.
After 28 days of immunization and at baseline, the serum neutralizing to live SARS-CoV-2 was performed. The primary outcomes of the study included geometric mean titer (GMT) and neutralization antibody seroconversion at 28 days following the 2nd dose. Collected data were evaluated using logistic regression analysis, chi-square test and analysis of variance (ANOVA).
In total, about 809 people participated which were categorized in 3 vaccination groups: 0-14 (n=270), 0-21 (n=270) and 0-28 (n=269) and randomized to receive 2 doses of injections. Following 28 days of the second injection, GMT and seroconversion rates of the SARS-CoV-2 neutralizing antibody were observed to be as follows (Table 1):
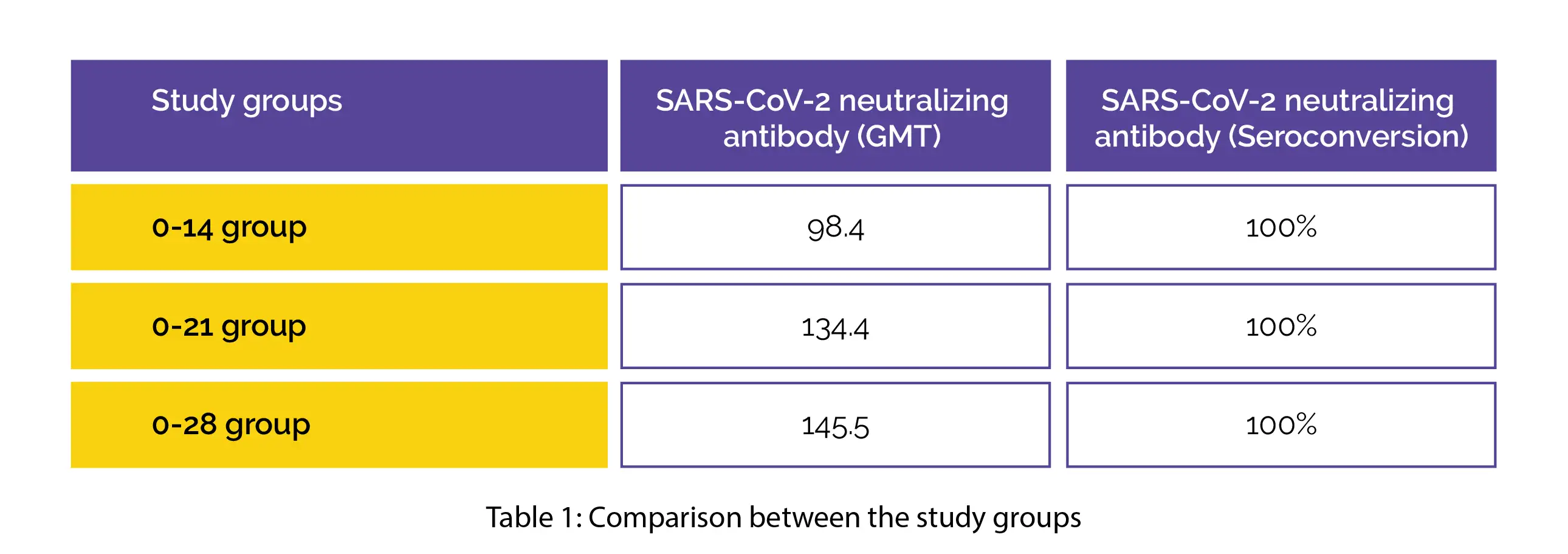
Similar results were noted in the intention-to-treat assessment. Mild adverse events were noted. These findings showed significant improvement in the SARS-CoV-2 neutralizing antibody levels, suggesting the need to optimize the vaccination strategy of the inactivated COVID-19 vaccine in high-risk occupational populations.
Infectious Diseases of Poverty
Safety and immunogenicity of inactivated SARS-CoV-2 vaccine in high-risk occupational population: a randomized, parallel, controlled clinical trial
Yongliang Feng et al.
Comments (0)