Categories
Change Password!
Reset Password!


In CRSsNP patients, an exhalation delivery system with Fluticasone relieves both sinus opacification and symptoms.
Exhalation Delivery System with Fluticasone (EDS-FLU) substantially improved sinus opacification and symptoms of chronic rhinosinusitis without nasal polyps (CRSsNP) compared to EDS-placebo in a 24-week study. In order to manage CRSsNP, researchers sought to compare the impact of EDS-FLU versus EDS-placebo twice daily.
The average percentages of CT-opacified volume across ethmoid/maxillary sinuses (APOV; week 24) and combined symptom score (CSS; week 4) were the co-primary endpoints ascertained. Patient-reported Global Impression of Change (PGIC), Sleep (PSQI), and Quality-of-Life (SNOT-22, SF-36) were the other outcomes ascertained.
In this phase 3 randomized controlled study, the baseline values of mean CSS= 6.0 and APOV= 62.0% indicated moderate-severe illness. Compared to EDS-placebo (n = 75), both EDS-FLU doses—186µg and 372µg—significantly decreased symptoms and sinus opacification: CSS least-square (LS) mean alteration and APOV LS mean alteration in EDS-placebo, EDS-FLU 186µg, and EDS-FLU 372µg is depicted in Table 1:
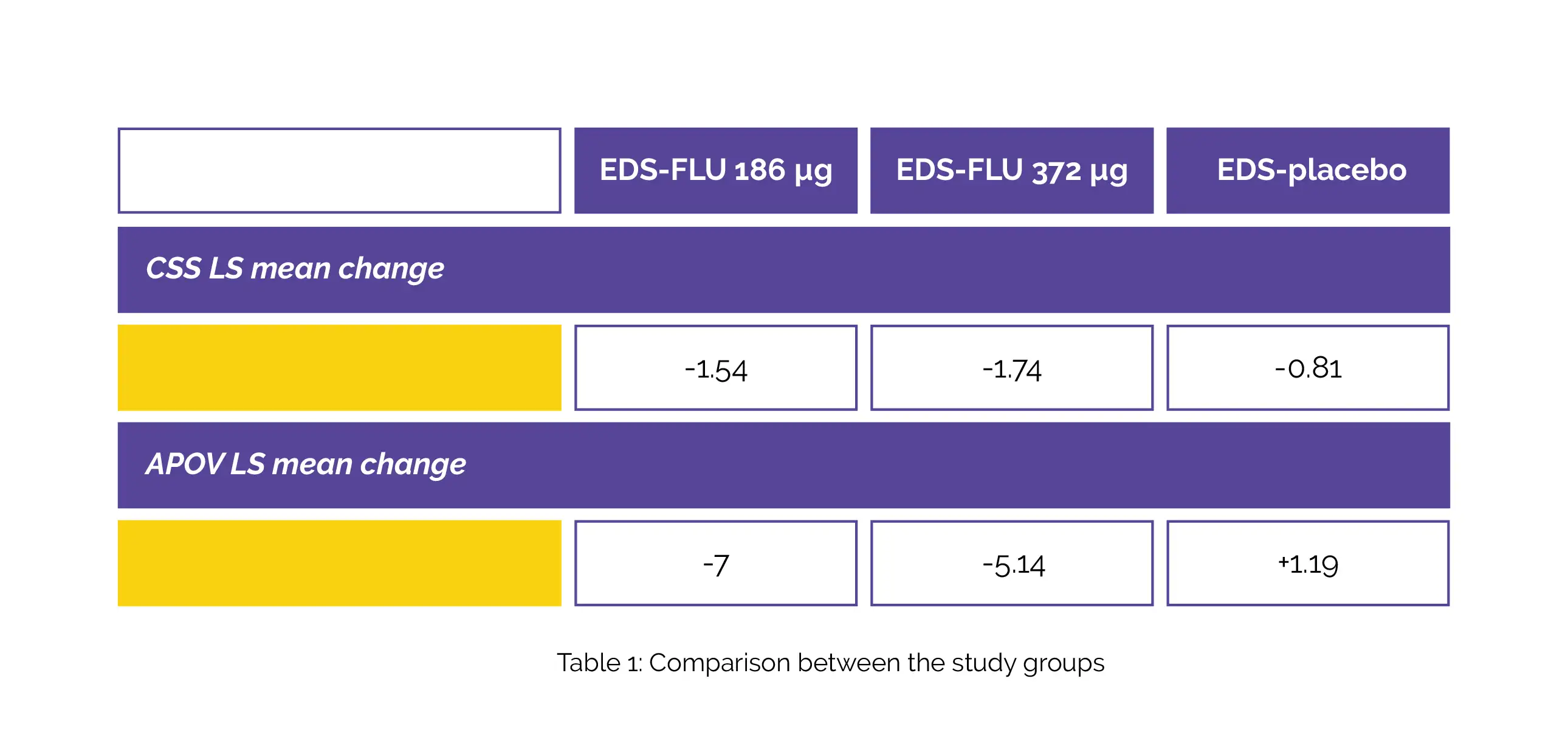
At Week 24, EDS-FLU (n = 145) versus EDS-placebo (n = 75) also substantially enhanced secondary outcomes such as PGIC (60% versus 25% much/very much improved), PSQI Global score (LS mean -1.54 versus -.33), SF-36v2 (LS Mean PCS 4.9 versus 1.8), and SNOT-22 (LS mean -17.5 versus -8.7). Depression, headache, epistaxis, and COVID-19 were the adverse events reported. Hence, the intranasal steroid in a device that supplies the drug to the regions above the inferior turbinate and behind the nasal valve is promising for CRSsNP management.
Annals of Allergy, Asthma & Immunology
RANDOMIZED, CONTROLLED TRIAL OF EXHALATION DELIVERY SYSTEM WITH FLUTICASONE FOR CHRONIC RHINOSINUSITIS WITHOUT NASAL POLYPS
A. Peters
Comments (0)