Categories
Change Password!
Reset Password!


In individuals with antihistamine-resistant chronic spontaneous urticaria, Dupilumab substantially improves symptoms.
In a randomized, placebo-controlled, 24-week, phase 3 trial, Dupilumab showed good tolerability and significant symptomatic improvements in people with urticaria refractory to antihistamines. Investigators sought to assess the safety and effectiveness of Dupilumab for the management of chronic spontaneous urticaria refractory to antihistamine therapy.
Subjects (≥ 6 years) receiving a standard or ≤ 4-fold antihistamine dosage were randomly allocated to get either add-on Dupilumab (n = 70) in doses of 300 mg for adolescents/adults ≥ 60 kg and 200 mg for children ≥ 30 kg and adolescents < 60 kg, or matching placebo (n = 68) which was administered subcutaneously every two weeks. Alteration from the baseline in the Urticaria Activity Score over 7 Days (UAS7) and the Itch Severity Score over 7 Days (ISS7) at week 24 were major outcomes ascertained.
Alteration from baseline in the Hive Severity Score over 7 days (HSS7) at Week 24 was the secondary outcome ascertained. Table 1 shows the mean ISS7, UAS7, and HSS7 (Dupilumab/placebo) at baseline and week 24.
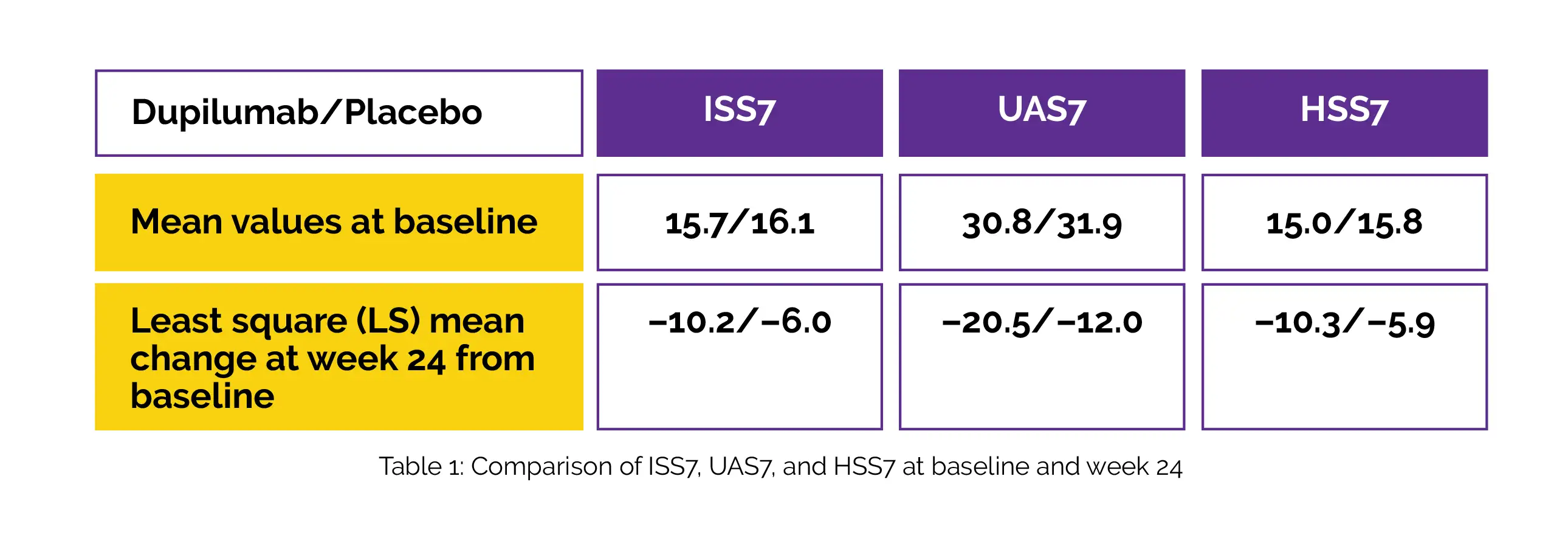
Overall, treatment-emergent adverse events (TEAEs) were similar between Dupilumab and placebo: 35 (50.0%)/40 (58.8%); incidence of injection site reactions was 8 (11.4%)/9 (13.2%), conjunctivitis 0/1 (1.5%), and serious TEAEs 2 (2.9%)/5 (7.4%). Hence, the monoclonal antibody Dupilumab was beneficial in patients with urticaria who remained symptomatic despite the use of standard-of-care antihistamines.
The Journal of the American Academy of Dermatology
33004 Dupilumab significantly reduces itch and hives in patients with chronic spontaneous urticaria (CSU) who remain symptomatic despite use of standard-of-care antihistamines: Results from a phase 3 trial (LIBERTY-CSU CUPID study A)
Marcus Maurer et al.
Comments (0)