Categories
Change Password!
Reset Password!


In terms of efficacy or safety, DA-2802 was not inferior to tenofovir disoproxil fumarate in patients suffering from chronic hepatitis B.
A randomized controlled trial revealed DA-2802 (tenofovir disoproxil orotate) to be an effective and safe therapeutic option for people with chronic hepatitis B. Hyung Joon Kim et al. aimed to explore safety and effectiveness of DA-2802 compared to tenofovir disoproxil fumarate for chronic hepatitis B management.
In this double-blind clinical study, 123 subjects were randomly segregated to get tenofovir disoproxil fumarate at a dose of 300 mg once daily (n=61) or DA-2802 at a dose of 319 mg once daily (n=62) for 48 weeks. The major efficacy outcome was alteration in hepatitis B virus (HBV) DNA level at week 48 following dosing in comparison with baseline.
The percentage of people having undetectable HBV DNA, loss of hepatitis B surface antigen, normal alanine aminotransferase levels, and loss of hepatitis B envelop antigen (HbeAg) were the secondary efficacy outcomes. Investigation of adverse events was also done.
For efficacy assessment, 122 volunteers were incorporated into the full analysis set. Assessment of primary outcome utilizing nonparametric analysis of covariance revealed profound findings, that substantiated non-inferiority of DA-2802 when compared to tenofovir disoproxil fumarate by a prespecified noninferiority margin of 1.
The alteration in HBV DNA level at week forty-eight from baseline, the proportion of undetectable HBV DNA, the percentage of people who had normal levels of alanine aminotransferase, and the percentage of people with HBeAg loss in the study groups, are shown in Table 1:
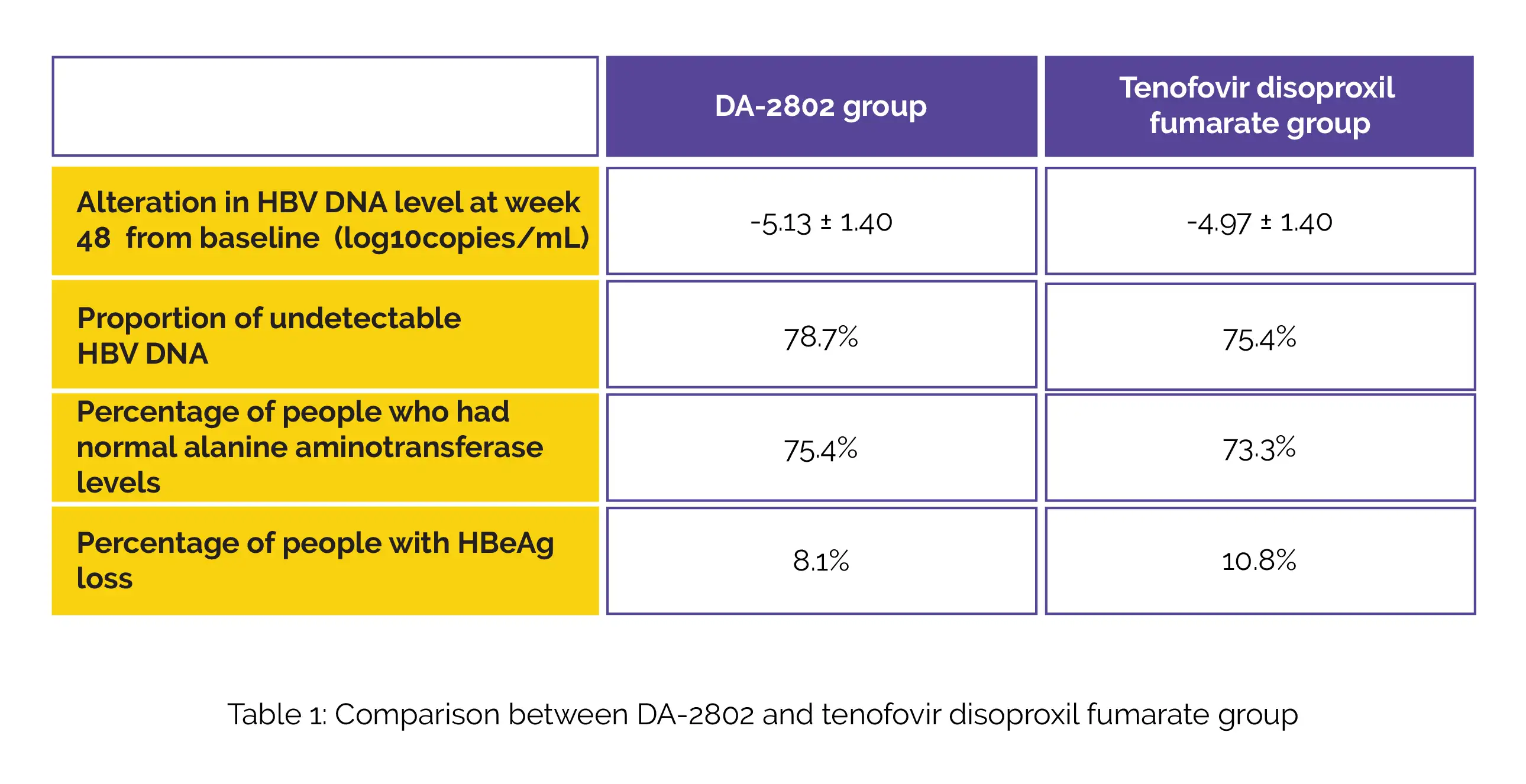
There was no loss of hepatitis B surface antigen in any of the participants. The frequency of side effects during therapy was noted to be similar between the study groups. Most side effects were found to be mild to moderate in severity. Thus, DA-2802 use appears to be beneficial for people diagnosed with chronic hepatitis B.
Journal of Korean Medical Science
A Multi-Center, Double-Blind Randomized Controlled Phase III Clinical Trial to Evaluate the Antiviral Activity and Safety of DA-2802 (Tenofovir Disoproxil Orotate) and Viread (Tenofovir Disoproxil Fumarate) in Chronic Hepatitis B Patients
Hyung Joon Kim et al.
Comments (0)