Categories
Change Password!
Reset Password!


In people suffering from COVID-19, convalescent plasma therapy along with standard medical therapy led to better clinical outcomes.
A phase-III randomized controlled study depicted that for improved outcomes in coronavirus-infected people, convalescent plasma therapy with adequate antibody titres must be administered in conjunction with standard medical therapy in the initial three days of hospitalization. Meenu Bajpai et al. aimed to investigate efficacy and safety of convalescent plasma with standard medical therapy in severe SARS-COV-2 infection.
In this multicentric, open-labelled trial (COPLA-II), 400 patients were segregated in the ratio of 1:1 into 2 groups: (a) Group 1 (n=200): Patients were given convalescent plasma transfusion + standard medical therapy, and (b) Group 2 (n=200): Patients were given standard medical therapy only. The time to clinical improvement estimated by a two-point decrease in ordinal scale was the primary endpoint ascertained. Occurrence of side effects, levels of cytokine, SARS-CoV-2 antibody levels, mortality, the percentage of patients on mechanical ventilation on day 7, and O2 therapy duration was the secondary outcome ascertained.
In the study groups, the median time to a two-point decrease in ordinal scale was found to be 9 days (Interquartile Range [IQR]=7-13). The median duration of O2 therapy was less in the convalescent plasma group when compared to standard medical therapy, as shown in Table 1:
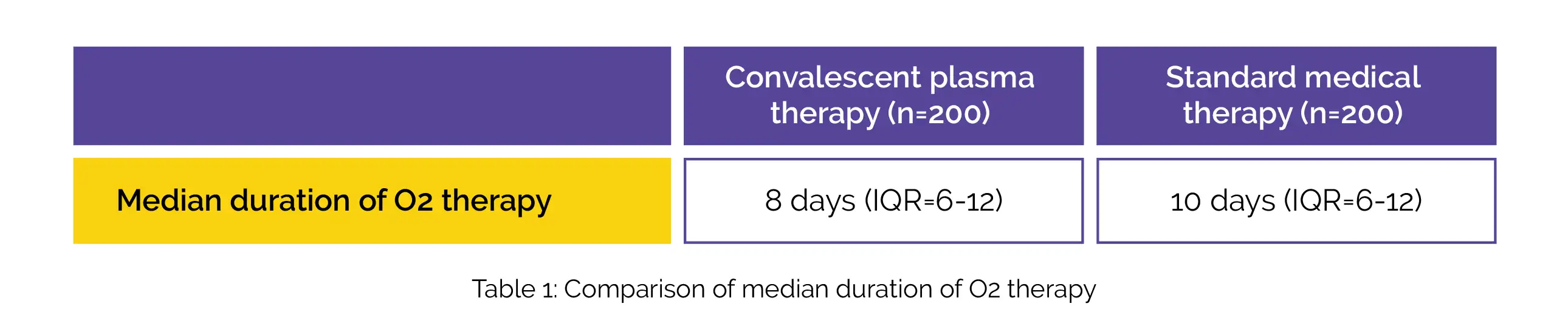
In the convalescent plasma group, a considerable improvement was seen in the partial pressure of oxygen/fractional inspired oxygen (PaO2/FiO2) ratio at 7 days. Till 28 days, no difference was observed in mortality in both the groups. A substantial drop in the ordinal scale was noted following the administration of convalescent plasma within 3 days of admission to the hospital. In the convalescent plasma group, the neutralizing antibody titres at 48 hours were higher when compared to the standard medical therapy group.
With convalescent plasma therapy, a significant reduction in D-dimer at 7 days and levels of tumor necrosis factor-α at 48 hours was observed. In the convalescent plasma transfusion group, 3 (1.5%) patients experienced mild allergic reactions. Hence, this study substantiated the antiviral efficacy of convalescent plasma transfusions as an adjunct to the standard medical treatment in the initial days of COVID-19 infection.
BMJ Open
Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial)
Meenu Bajpai et al.
Comments (0)