Categories
Change Password!
Reset Password!


Budesonide + Loratadine effectively relieve allergic rhinitis symptoms and improve T lymphocyte subsets and nasal physiological function.
In comparison with Mometasone furoate nasal spray plus Loratadine tablets, the combination of Loratadine and Budesonide exhibited superior efficacy and safety for the management of allergic rhinitis and efficiently improved symptoms, nasal physiological function, and T lymphocyte subsets, as deciphered from a recent study. Researchers sought to evaluate the impact of Loratadine tablets combined with other medications on T lymphocyte subsets and the physiological function of the nasal passages in individuals with allergic rhinitis.
Overall, 120 people with allergic rhinitis were split into a control group and a research group at random. Loratadine tablets plus intranasal Mometasone furoate were administered to the control group, whereas Loratadine plus Budesonide was given to the research group. Between both the groups, the occurrence of adverse events, nasal physiological function, T lymphocyte subset, immune function indicators, length of clinical symptom remission, and effectiveness were compared.
The research group's effective rate was 96.67%, with significant effectiveness in 36 cases, effectiveness in 22 cases, and ineffectiveness in 2 cases. In contrast, the control group's effectiveness rate was 83.33%, with substantial effectiveness in 23 cases, effectiveness in 27 cases, and ineffectiveness in 10 cases. The effective rate in research group was greater than control group (χ2: 5.925), as shown in Figure 1:
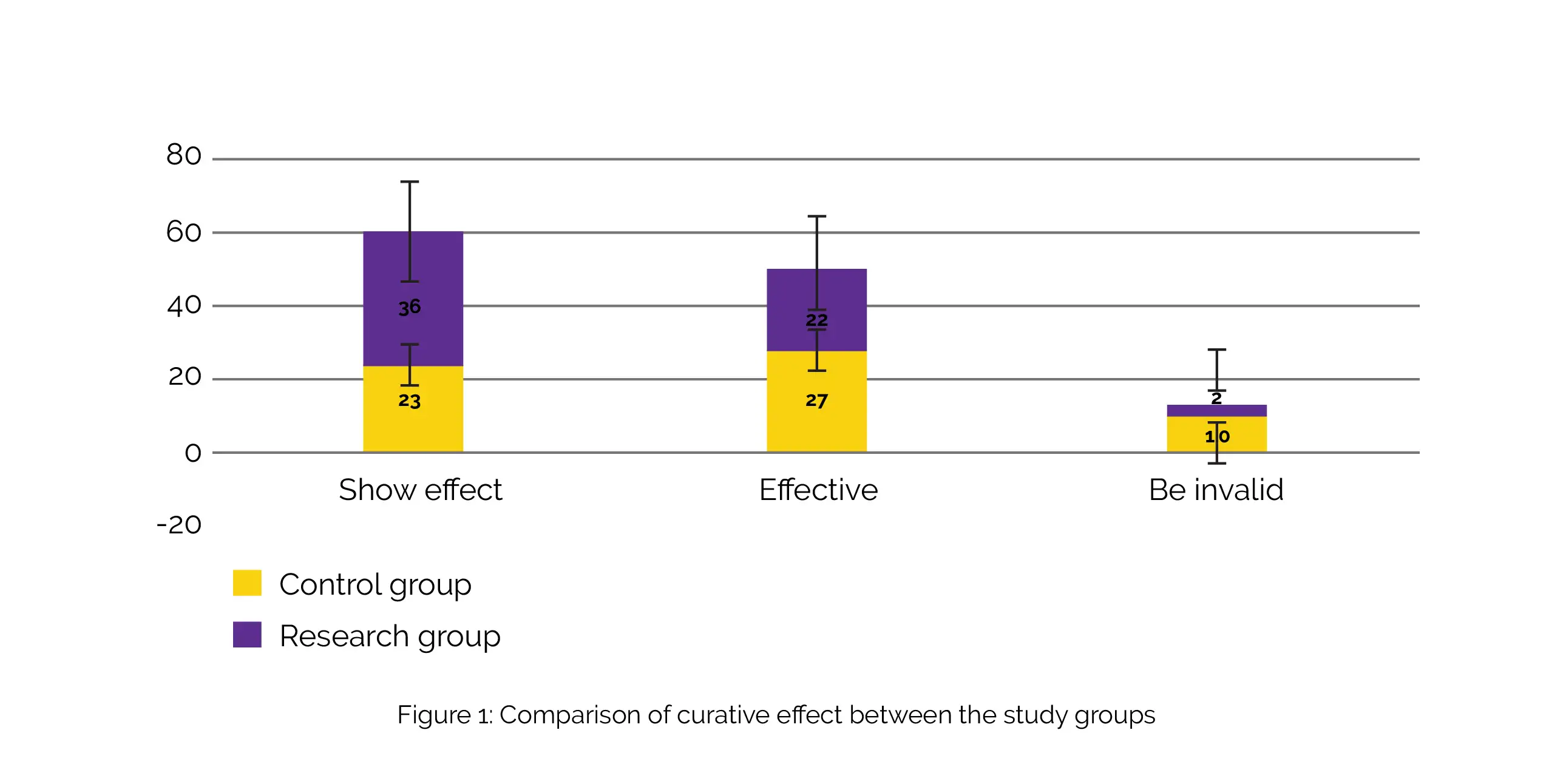
The research group's clinical symptom relief time for sneezing, itching, nasal congestion, and runny nose was shorter than that of control group, and difference was clinically meaningful. The research group exhibited greater Th1/Th2 and reduced Immunoglobulin E (IgE), Interleukin (IL)-8, and IL-6 when compared to the control group, as revealed by the comparison of immune function indicators.
Prior to nursing, no clinically meaningful differences were witnessed between the T lymphocyte subsets of both the groups. However, post-intervention, the T lymphocyte subsets of both groups reduced. After treatment, the level of cluster of differentiation (CD)4+/CD8+, CD4+, CD8+, CD4+, and CD3+ lymphocytes in research group was reduced than control group. These differences were clinically meaningful.
Nasal physiological function of both the groups did not differ significantly prior to intervention, but it improved thereafter. The mucosal ciliary clearance rate, nasal resistance, and mucosal ciliary transit time of the research group were significantly reduced in comparison with the control group. Compared to the control group, the occurrence of adverse effects in the research group was substantially reduced. Hence, Budesonide along with Loratadine appears to be safe and efficacious in the treatment of allergic rhinitis.
Computational Intelligence and Neuroscience
Effect of Loratadine Tablets in Combination with Other Drugs on Nasal Physiological Function and T Lymphocyte Subsets in Patients with Allergic Rhinitis
Jie Zhang et al.
Comments (0)