Categories
Change Password!
Reset Password!


For H. pylori rescue therapy, Rifabutin triple therapy can serve as a viable substitute to the traditional Bismuth quadruple therapy with fewer adverse effects and better compliance.
Investigators have made a groundbreaking discovery in the fight against Helicobacter pylori (H. pylori). A recent noninferiority study has shown that Rifabutin-containing triple therapy could provide a new and highly successful approach for H. pylori-infected individuals who have previously failed multiple treatment attempts. Investigators examined the effectiveness and safety of Rifabutin triple therapy and Bismuth quadruple therapy.
Conducted between May 2021 and October 2022, this randomized controlled trial involved 364 subjects with persistent H. pylori infections. The participants were divided into two groups. The first group received Rifabutin-containing triple therapy, consisting of 14-day Esomeprazole (20 mg), Amoxicillin (1.0 g), and Rifabutin (150 mg) taken twice daily. On the other hand, the second group received bismuth quadruple therapy, which included Esomeprazole (20 mg) and Bismuth (220 mg) taken twice daily, along with Metronidazole (400 mg) and Tetracycline (500 mg) taken four times daily.
Using agar dilution and E-test methods, antimicrobial susceptibility was examined. Table 1 summarizes H. pylori eradication rates for both Rifabutin triple therapy group and the Bismuth quadruple therapy group according to intention-to-treat, per-protocol, and modified intention-to-treat analysis.
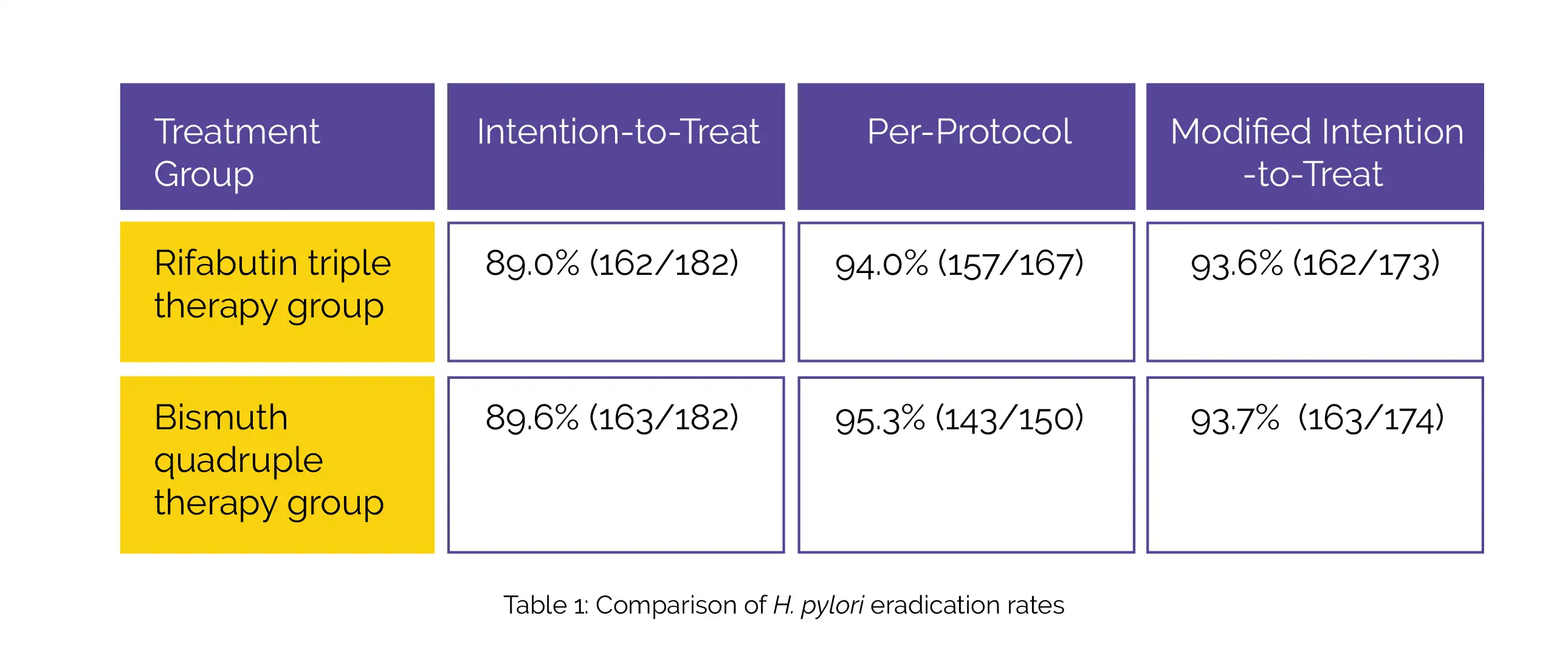
In conclusion, Rifabutin triple therapy is a safe and effective rescue treatment option. It offers the advantage of fewer side effects and higher compliance rates among patients, which is crucial for the successful eradication of the infection.
The Journal of Infectious Diseases
Rifabutin-Containing Triple Therapy Versus Bismuth Quadruple Therapy for Helicobacter pylori Rescue Treatment: A Multicenter, Randomized Controlled Trial
Jinnan Chen et al.
Comments (0)