Categories
Change Password!
Reset Password!


Olamkicept 600 mg is an effective initial therapy for patients with ulcerative colitis.
Compared to Olamkicept 300 mg, biweekly use of Olamkicept 600 mg intravenously led to a superior clinical response at 12 weeks in patients with active ulcerative colitis, a study published in JAMA Network explained. This randomized controlled trial by Shenghong Zhang and colleagues examined the effect of Olamkicept (selective inhibitor of soluble interleukin 6 [sIL-6R]/IL-6 complex) as induction therapy for ulcerative colitis.
A total of 91 adults (mean age of 41 years; 27.5% women) with ulcerative colitis and an inappropriate response to conventional therapy were considered. The study duration was February 2018 to December 2020 performed at 22 clinical study locations in East Asia. Suitable patients were randomized to receive intravenous Olamkicept 600 mg (30 patients) or Olamkicept 300 mg (31 patients) or placebo (30 patients) twice weekly for 12 weeks.
The clinical response (based on total Mayo score; range: 0 [normal]-12 [worst] at the starting) at week 12 was considered as the primary endpoint. Twenty-five secondary efficacy outcomes, together with clinical remission and mucosal healing at week 12 were considered as the secondary endpoints.
Out of the total, 79 (86.8%) accomplished the study. At week 12, more patients receiving Olamkicept 600 mg or 300 mg achieved clinical response than placebo, with adjusted difference vs placebo of 26.6% for 600 mg and 8.3% for 300 mg. Details of the clinical response achieved at week 12 are shown in the following Table 1:
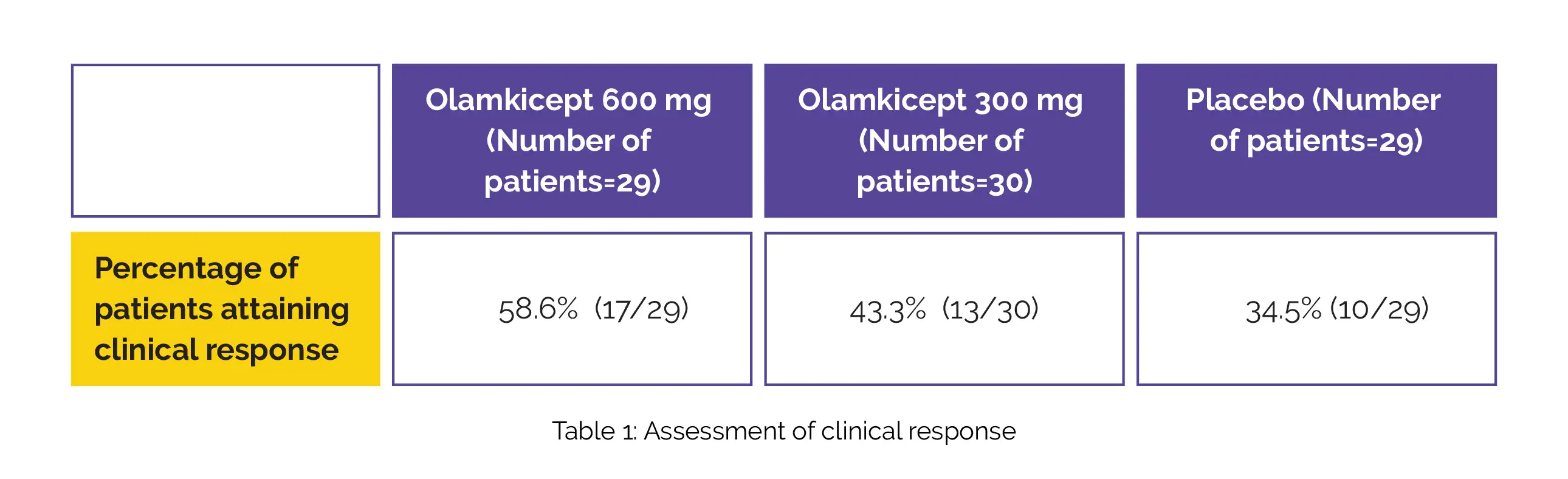
Sixteen out of 25 secondary outcomes were statistically noteworthy in patients who received Olamkicept 600 mg compared with placebo. Among participants randomly allocated to get 300 mg, 6 of 25 secondary outcomes were statistically significant in comparison with placebo. The percentage of people experiencing treatment-related adverse events is shown in Table 2:
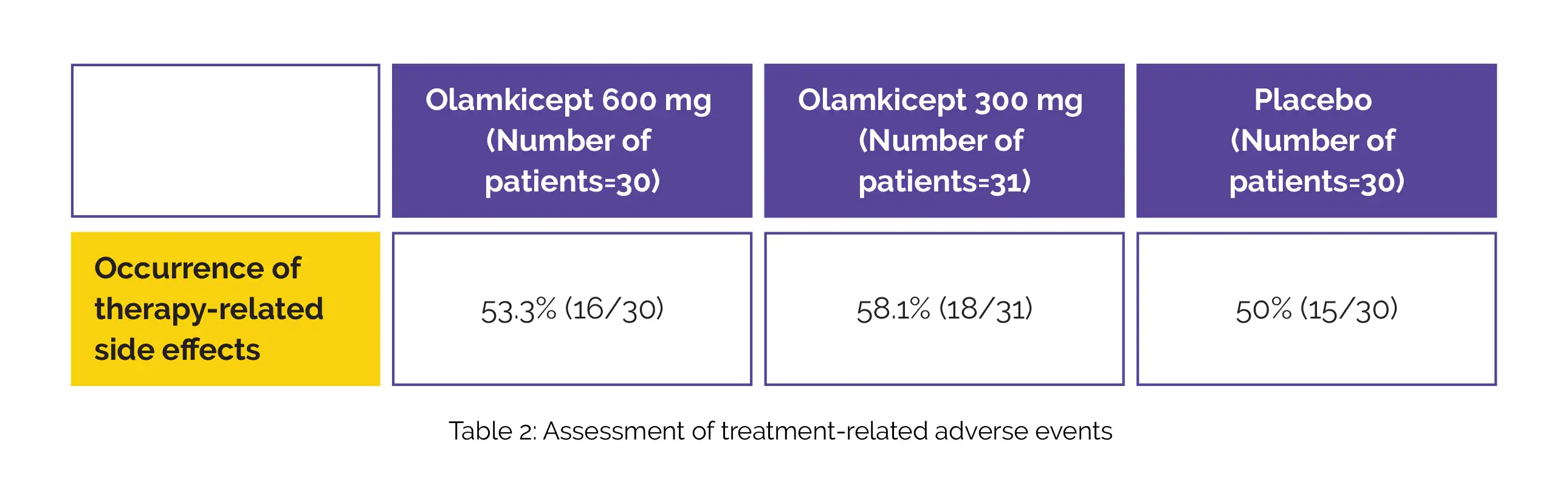
In comparison with the placebo, patients in the Olamkicept groups had the presence of bilirubin in their urine, and raised uric acid and aspartate aminotransferase levels (drug-related adverse events). Hence, intravenous Olamkicept 600 mg twice weekly raises the probability of clinical response at 12 weeks in ulcerative colitis-affected patients. Future research to assess longer-term efficacy and safety is awaited.
JAMA Network
Effect of Induction Therapy With Olamkicept vs Placebo on Clinical Response in Patients With Active Ulcerative Colitis
Shenghong Zhang et al.
Comments (0)