Categories
Change Password!
Reset Password!


The H. pylori elimination rate of high-dose dual therapy is comparable to bismuth-containing quadruple therapy in H. pylori-infected treatment-naive people.
A prospective, open-label, multicenter, randomized controlled trial depicted that for Helicobacter pylori (H. pylori) elimination, high-dose dual therapy exhibited comparable effectiveness and compliance, fewer side effects, and reduced costs when compared to bismuth-containing quadruple therapy for treatment-naive people infected with H. pylori.
For comparing two different therapies, 700 participants were randomized to get high-dose dual therapy (20 mg esomeprazole 4 times daily and 1000 mg amoxicillin 3 times daily for fourteen days, n=350) or bismuth-containing quadruple therapy (20 mg esomeprazole, 1000 mg amoxicillin, 500 mg clarithromycin, and 220 mg bismuth potassium citrate all twice daily for fourteen days, n=350).
A comparison of efficacy, costs, adverse events, and patient compliance was done. Both the groups attained comparable H. pylori elimination rates. Compared to the quadruple therapy group, the high-dose dual therapy group exhibited a reduced rate of adverse events and reduced costs. Both the study groups revealed satisfactory compliance, as illustrated in Table 1:
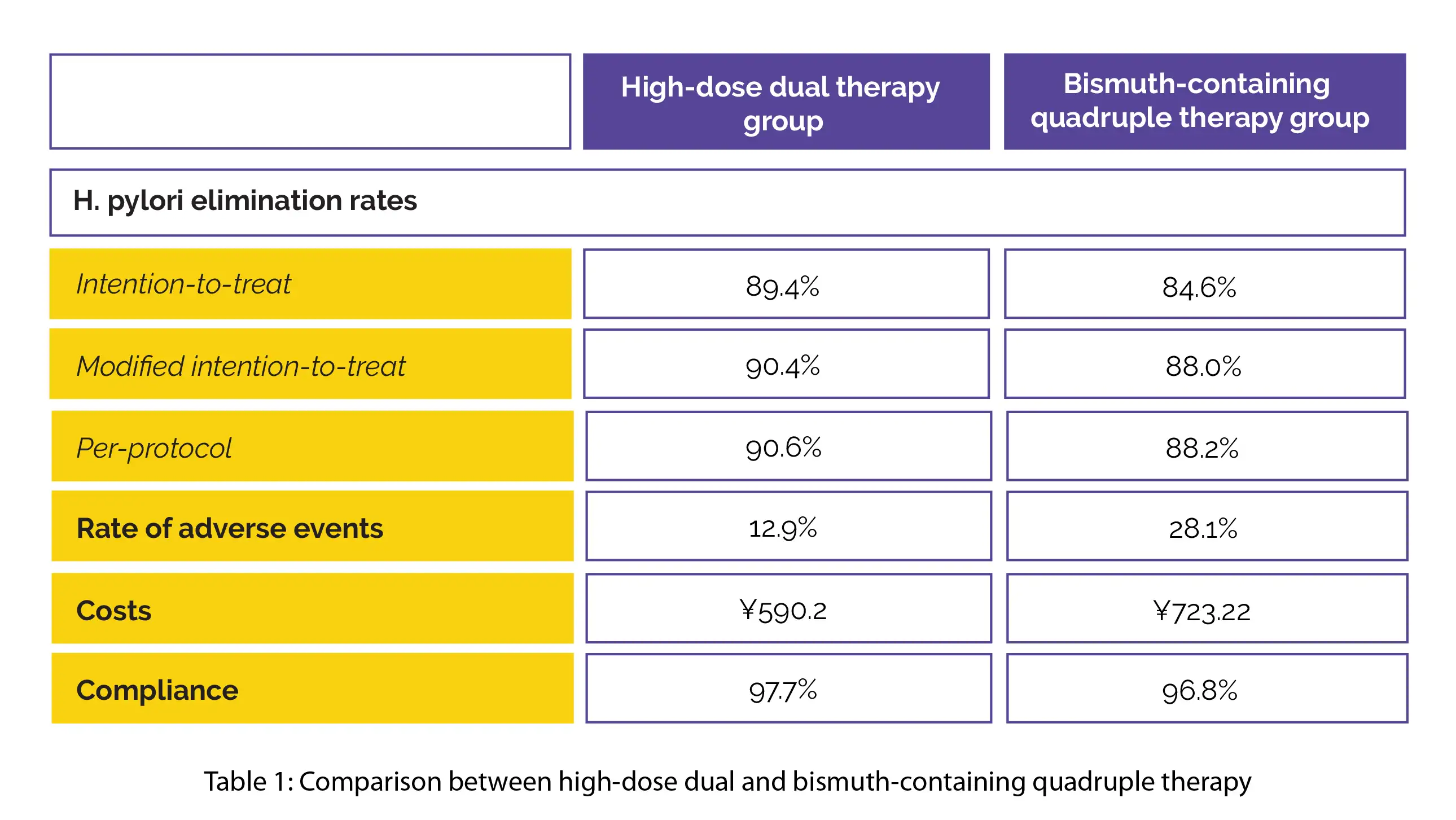
Thus, bismuth-containing quadruple therapy and high-dose dual therapy have similar compliance and effectiveness in H. pylori-infected treatment-naive people.
Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy
Comparison of high-dose dual therapy with bismuth-containing quadruple therapy in Helicobacter pylori-infected treatment-naive patients: An open-label, multicenter, randomized controlled trial
Jia-Lun Guan et al.
Comments (0)