Categories
Change Password!
Reset Password!


Baricitinib + standard of care exhibited a profound effect on all-cause mortality reduction at 28 days in COVID-19 patients, with the effect being maintained at 60 days.
In hospitalized adults suffering from SARS-CoV-2 infection and who were receiving extracorporeal membrane oxygenation or invasive mechanical ventilation, baricitinib + standard of care therapy remarkably decreased mortality when compared with placebo + standard of care therapy. It showed consistency with the mortality decrease noted in less severely ill people in the hospitalized primary COV-BARRIER trial population.
Following the study design of phase III trial (COV-BARRIER) in a critically ill cohort not incorporated in the main phase III clinical trial, investigators undertook this study to assess safety and efficacy of baricitinib (oral, selective Janus kinase 1/2 inhibitor) + standard of care (predominantly included corticosteroids) in coronavirus-infected people (aged ≥18 years). The study included 101 critically ill volunteers hospitalized with lab-confirmed coronavirus disease and who needed oxygenation or ventilation.
The volunteers were randomized to receive 4 mg baricitinib (n=51) or placebo (n=50) once daily for up to fourteen days plus standard of care therapy. In this exploratory trial, the prespecified outcomes ascertained were: (i) Time to recovery through day 28, (ii) All-cause mortality through days 28 and 60, (iii) Number of ventilator-free days, and (iv) Duration of hospital stay.
The safety assessment was performed in the safety population while the efficacy assessment was performed in the intention-to-treat population. The standard of care therapy included baseline systemic corticosteroid usage in 87 (86%) volunteers. In comparison with placebo, baricitinib therapy led to a substantial decline in 28-day all-cause mortality and 60-day mortality, as shown in Table 1:
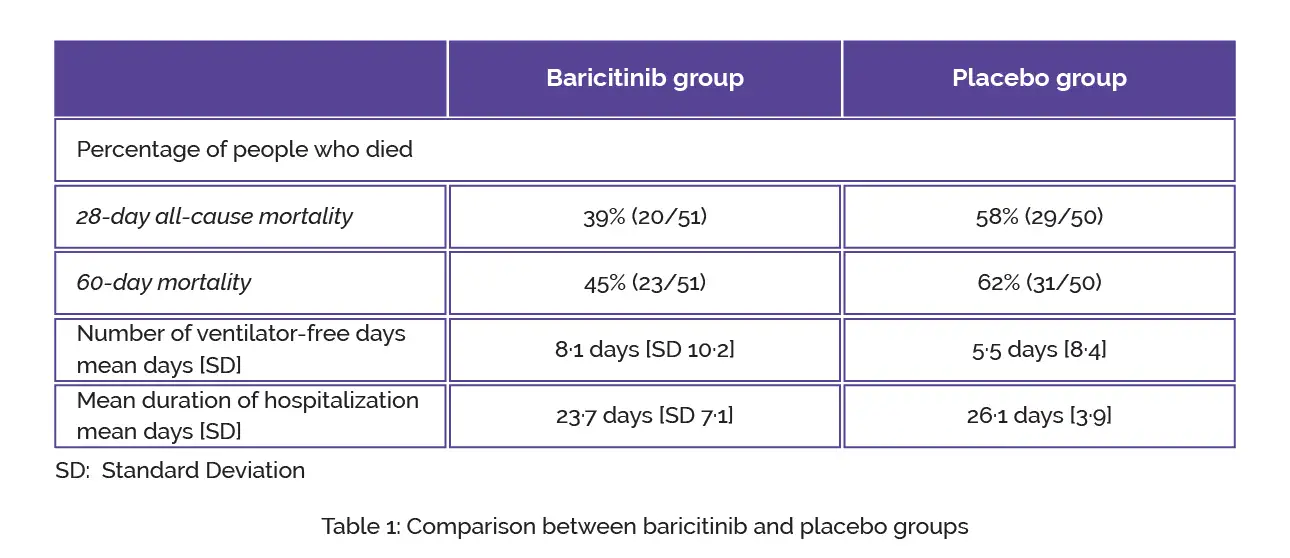
In every 6 baricitinib-treated volunteers, 1 additional death was prevented when compared to placebo at day 28 and day 60. Regarding the number of ventilator-free days, no inter-group difference was witnessed. The mean duration of hospitalization in baricitinib-treated people was not considerably shorter when compared to placebo-treated subjects.
Adverse cardiovascular events, blood clots, and rates of infections were comparable between the treatment groups. But, this was an exploratory study having a relatively small sample size. Thus, further phase 3 trials are warranted for confirming these findings.
The Lancet Respiratory Medicine
Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial
E Wesley Ely et al.
Comments (0)