Categories
Change Password!
Reset Password!


Dose of Abrocitinib can be escalated to 200 mg for enhanced symptom relief in people with moderate to severe atopic dermatitis, with minimal impact on platelet counts.
Research in 'Dermatology and Therapy' aimed to predict the early efficacy of Abrocitinib in atopic dermatitis, suggesting potential for early dose adjustments based on week 4 responses with Abrocitinib 100 mg per day.
April W. Armstrong et al. performed a post-hoc analysis of different trials of Abrocitinib for platelet counts and simulations. A method of training and validation was utilized to identify the factors that predict response at week 4. This was done using the Investigators Global Assessment (IGA) scale, Eczema Area and Severity Index (EASI), Peak Pruritus Numerical Rating Scale (PP-NRS) for estimating the itch severity, and the percentage change in EASI from the start. The week 12 outcomes were evaluated using EASI-75 improvement and IGA scores of 0 (as clear) or 1 (almost clear) for lesions, with a ≥ 2-point improvement from the starting point.
The outcomes for the training group (number of patients = 453) based on EASI-50 responders and non-responders at week 4 with Abrocitinib 100 mg, showing the percentages of EASI-75 and IGA responses at week 12, are provided in Table 1 below:
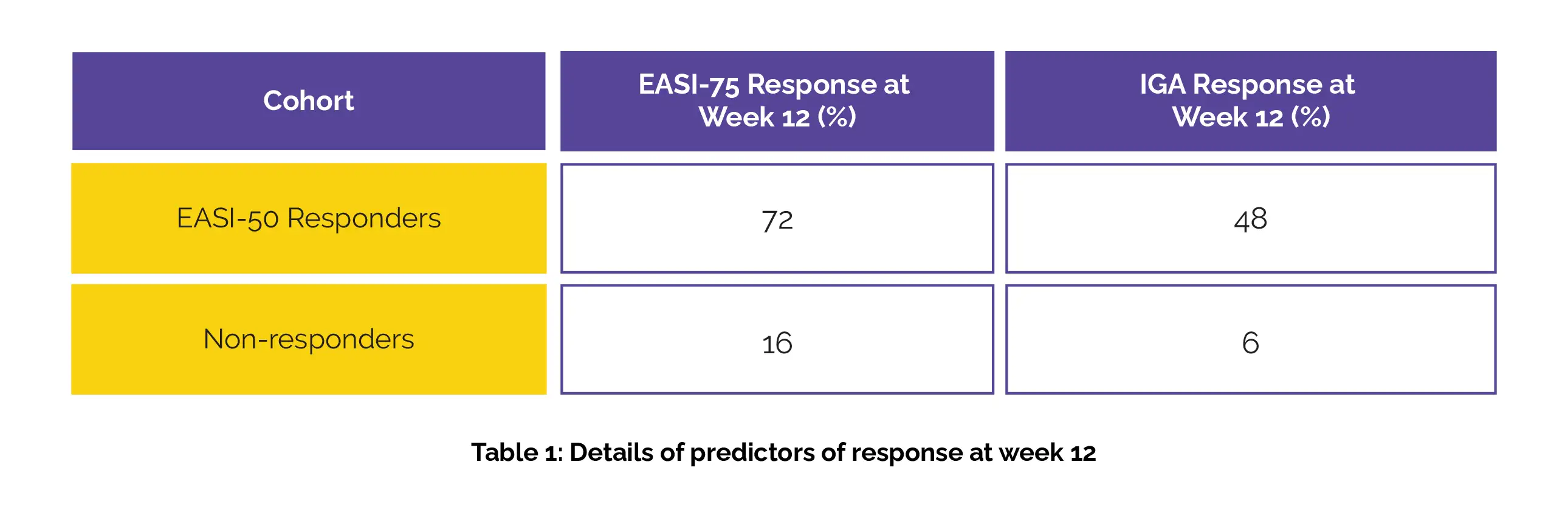
The results were comparable for those who had a response at week 4, whether based on an IGA score of 2, a 4-point improvement from baseline in PP-NRS, or an EASI scored 8, compared to those who did not respond. Platelet counts following an increase in Abrocitinib dosage from 100 mg to 200 mg were consistent with counts observed with continuous dosing of both 100 mg and 200 mg of Abrocitinib.
Summing up, atopic dermatitis patients responding to Abrocitinib 100 mg at 4 weeks showed continued relief at 12 weeks. Increasing to 200 mg helped non-responders; temporary platelet decreases at week 4 normalized by week 12, indicating manageable safety with dose adjustment.
Dermatology and Therapy
Predicting Abrocitinib Efficacy at Week 12 Based on Clinical Response at Week 4: A Post Hoc Analysis of Four Randomized Studies in Moderate-to-Severe Atopic Dermatitis
April W. Armstrong et al.
Comments (0)