Categories
Change Password!
Reset Password!


Xylometazoline hydrochloride (0.1%) nasal decongestant spray effectively alleviates symptoms post-septoplasty, speeds recovery, boosts patient satisfaction, and has no adverse effects, supporting its use as a therapeutic adjunct.
Xylometazoline-hydrochloride remarkably outperformed saline nasal spray in an article featured in the “Ear Nose Throat Journal” assessing the efficacy of 0.1% Xylometazoline-hydrochloride nasal spray versus 0.9% isotonic-saline spray for post-septoplasty symptom relief. This randomized clinical trial involved 120 septoplasty patients.
The enrolled subjects were randomly assigned to get either saline or Xylometazoline for one week (administered twice daily, with a 12-hour interval between doses, as a single spray in each nostril), with follow-ups on days 3 and 7 post-surgery. The Chi-square test was employed to compare postoperative symptoms, nasal endoscopic findings, adverse effects, and patient satisfaction, with a P value of < 0.05 deemed significant.
Out of 120 participants, 106 were analyzed, with 53 in each group. By day 3, the Xylometazoline group reported markedly lower rates of self-reported symptoms when compared to the saline group. By day 7, the Xylometazoline group showed no self-reported symptoms and clinical nasal findings, while the control group continued to experience moderate symptoms. Additionally, the Xylometazoline group reported remarkably higher patient satisfaction, as shown in Table 1 below.
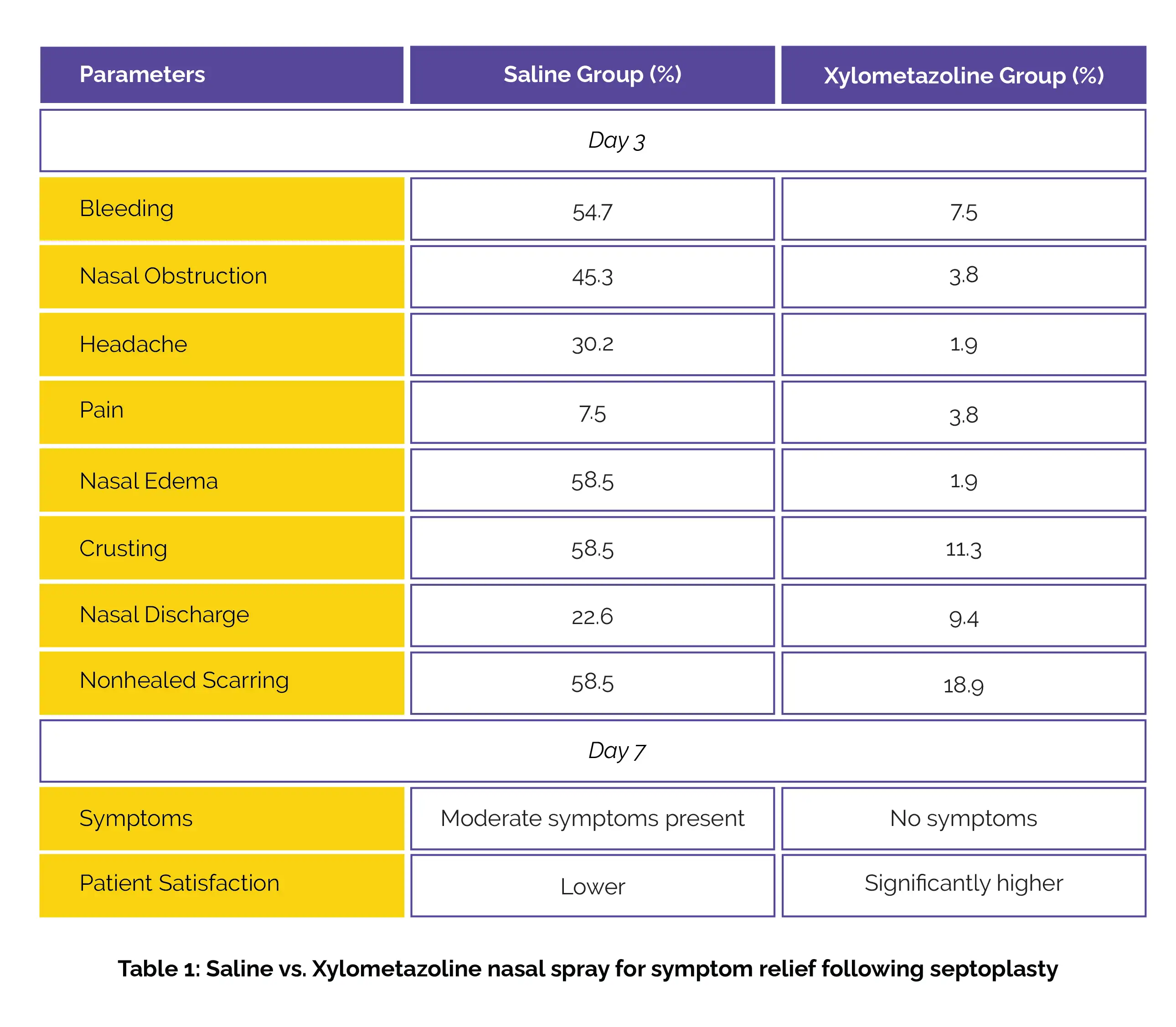
Thus, the use of Xylometazoline nasal spray is beneficial for alleviating clinical nasal findings and symptoms after septoplasty, without any adverse effects. This indicates its potential for expanded clinical application.
Ear Nose Throat Journal
Efficacy of 0.1% Xylometazoline-Hydrochloride Nasal Decongestant Spray in Postoperative Sign/Symptom Relief Following Septoplasty: A Randomized Control Trial
Muhammad Hamza Dawood et al.
Comments (0)