Categories
Change Password!
Reset Password!


Ublituximab, a low-fucose monoclonal antibody, that targets a unique epitope on the cluster of differentiate 20 (CD20) is used to treat relapsing forms of multiple sclerosis.
Ublituximab, a low-fucose monoclonal antibody, that targets a unique epitope on the cluster of differentiate 20 (CD20) is used to treat relapsing forms of multiple sclerosis. [1,2] With a molecular weight of 147,000 Daltons, this large protein molecule is the first and only anti-CD20 monoclonal antibody to combat multiple sclerosis. [2,3] FDA authorized it in December 2022 to relieve the relapsing forms of multiple sclerosis that may be administered in a one-hour infusion two times a year after the initial dosage.
Pharmacological Class: Anti-CD20 monoclonal antibody [2]
In adults, the glycoengineered antibody Ublituximab is indicated to combat the relapsing forms of multiple sclerosis, like
Pathogenesis of several cancers and autoimmune diseases like myelin oligodendrocyte glycoprotein immunoglobulin G (IgG)-associated disease, neuromyelitis optica spectrum illness, and multiple sclerosis are mediated by B-cell dysregulation. A fraction of CD3-positive T cells, pre-B cells, memory B cells, and mature/immature B cells, all express the antigen CD20. The anti-CD20 antibodies can hence cause Fc-gamma receptor (FcγR)-induced phagocytosis (antibody-dependent cellular cytotoxicity [ADCC]), activation of complement pathways, and B-cell depletion by direct cell mortality. Despite the development of various anti-CD20 antibodies, treatment has been impeded by low expression of CD20 by malignant cells in illnesses like B-cell chronic lymphocytic leukemia and suboptimal antibody-dependent cytotoxicity.
In contrast to other approved antibodies including ocrelizumab, obinutuzumab, ofatumumab, and rituximab, Ublituximab binds to an epitope on CD20 with a comparable binding constant to rituximab. To improve Ublituximab's interaction with FcγR, particularly FcγRIIIA (CD16) expressed by macrophages and natural killer cells, it is generated in the rat YB2/0 cell line with a low fucose concentration (24% as opposed to 93% for rituximab).
Because of this distinction, Ublituximab has improved ADCC, including for malignant cells with minimal CD20 expression. Ublituximab's exact mechanism of action in treating multiple sclerosis is uncertain, but it is assumed to entail CD20 binding and consequent cell lysis. [2]
(a) 150 mg intravenous infusion for the initial infusion
(b) Two weeks following the initial infusion, provide a second intravenous infusion of 450 mg
(c) 450 mg intravenous infusions 24 weeks following the initial infusion, and then every 24 weeks thereafter for subsequently administered infusions.
Absorption
The mean Cmax was 139 mcg/mL while and the geometric mean steady-state area under the curve (AUC) was 3000 mcg/mL per day after administration of the authorized suggested dose of Ublituximab. This drug's exposure rises proportionally throughout a dosage range of 150 mg to 600 mg in relapsing multiple sclerosis-affected people.
Volume of distribution
3.18 L is Ublituximab's estimated central volume of distribution.
Protein binding
Not Available
Metabolism
Non-specific proteolytic enzymes most likely metabolize Ublituximab to smaller peptides and amino acids.
Route of elimination
Not Available
Half-life
Ublituximab has a 22-day estimated mean terminal half-life.
Clearance
Not Available [2]
It is contraindicated in people with:
The risk of infection may rise when Ublituximab is used concurrently with other immunosuppressant or immune-modulating medications, such as immunosuppressant doses of corticosteroids. While co-administering immunosuppressive medications with Ublituximab, the possibility of additive immune system effects must be taken into account.
When transitioning from therapies with immune effects, the mechanism of action and duration of these medicines should be considered due to the potential additive immunosuppressive effects while starting Ublituximab. [5]
Serious side effects of Ublituximab include:
(a) Low immunoglobulins
Ublituximab may cause a decline in some kinds of antibodies.
(b) Infections
(c) Infusion reactions
Safety, efficacy, and tolerability of Ublituximab to treat relapsing forms of multiple sclerosis
In phase 2, placebo-controlled study by Fox E et al, Ublituximab infusion was safe, well-tolerated, and produced strong B-cell depletion, and a significant decrease in magnetic resonance imaging (MRI) relapses and activity. Participants were divided into six dosing groups and were given 3 Ublituximab infusions (150 mg over 1 to 4 hours on 1st day and 450-600 mg over 1-3 hours on the 15th day and week twenty-four). B-cell depletion was the major outcome ascertained.
By week 4, the median B-cell depletion in all cohorts (n = 48) was >99% and remained stable at weeks 24 and 48. In weeks 24 and 48, no T1 gadolinium-enhancing lesions were noted, and the volume of the T2 lesions had decreased by 10.6%. The study's annualized recurrence rate was 0.07, and 93% of participants were relapse-free (Figure 1).
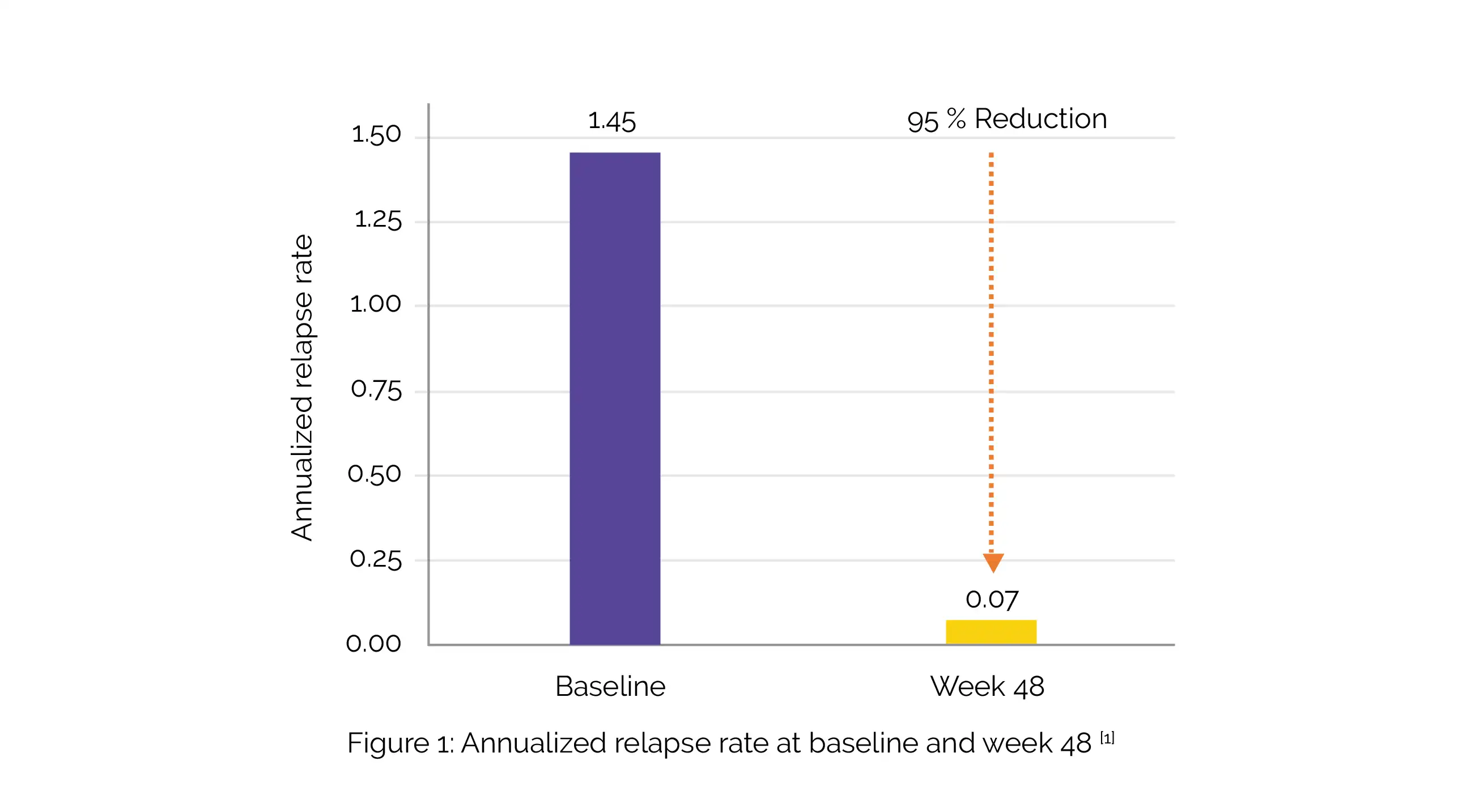
In the study, 74% of patients exhibited no evidence of disease activity. The majority of adverse events were infusion-associated reactions (all grade 1-2), without any apparent increase in frequency at the shorter infusion periods. No discontinuations due to adverse events were witnessed.
In conjunction with the prespecified 12- and 24-week confirmed disability improvement analyses, post-hoc investigations of sustained 12-week confirmed disability betterment, 9-Hole Peg Test (9-HPT), and Timed 25-Foot Walk (T25FW) yielded additional evidences of significant disability improvement with Ublituximab in ULTIMATE I and II in a study by Cree B et al. [6]
Ublituximab vs. Teriflunomide for multiple sclerosis
In a study by Steinman L et al, subjects suffering from relapsing multiple sclerosis were segregated at random to get intravenous Ublituximab (150 mg on day 1 that was followed by 450 mg on 15th day and at weeks 24, 48, and 72) and oral placebo or oral Teriflunomide (14 mg once everyday) and intravenous placebo.
The annualized relapse rate served as the major outcome while the secondary endpoints were the number of gadolinium-enhancing lesions on MRI at 96 weeks and disability deterioration. Throughout the 96-week period, Ublituximab produced lower annualized recurrence rates and lesser brain lesions on MRI (but not lower risk of worsening of disability) in people with relapsing multiple sclerosis contrasted with Teriflunomide. [7]
A meta-analysis was conducted to estimate the effect size utilizing RevMan 5.4.1 for the MRI results, annualized recurrence rate, MRI outcomes and no evidence of disease activity two years after the start of the Ublituximab treatment. Cohen's d for annualized recurrence rate of -0.17 in favor of Ublituximab was found in 2 randomized controlled trials (N = 1094). Ublituximab outperformed Teriflunomide in MRI-tested, week 96 findings of T1 (Cohen's d = -0.43) and T2 (Cohen's d = -0.55) lesions. Participants in the Ublituximab group experienced lower disease activity (Odds Ratio = 3.33). [8]
1. Fox E, Lovett-Racke AE, Gormley M, Liu Y, Petracca M, Cocozza S et al. A phase 2 multicenter study of Ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Multiple Sclerosis Journal. 2021 Mar;27(3):420-9.
2. Ublituximab. Drug Bank. Accession Number DB11850. Available online from: https://go.drugbank.com/drugs/DB11850 [Last accessed on: 23 February 2023]
3. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Ublituximab. [Updated 2023 Jan 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK588740/. Bookshelf ID: NBK588740, PMID: 36701507
4. Faissner S, Gold R. Efficacy and Safety of Multiple Sclerosis Drugs Approved Since 2018 and Future Developments. CNS Drugs. 2022 Aug;36(8):803-817.
5. Ublituximab. FDA LABEL. Available online from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761238s000lbl.pdf [Last accessed on: 23 February 2023]
6. Cree B, Fox E, Hartung HP, Alvarez E, Qian P, Wray S et al. Disability Improvements With Ublituximab in Relapsing Multiple Sclerosis (RMS): Expanded Disability Status Scale (EDSS), 9-Hole Peg Test (9-HPT), and Timed 25-Foot Walk (T25FW) Evaluations From the Phase 3 ULTIMATE I and II Studies (P5-4.009). May 03, 2022; 98 (18 Supplement).
7. Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. The New England Journal of Medicine. 2022 Aug 25;387(8):704-714.
8. Mukhtar H, Yasmeen U, Siddiqa S, Sarfraz Z, Sarfraz A. Outcomes of Ublituximab compared to Teriflunomide for relapsing multiple sclerosis: A meta-analysis. Multiple Sclerosis and Related Disorders. 2022 Sep;65:104002.
Comments (1)