Categories
Change Password!
Reset Password!


Pivmecillinam (amdinocillin pivoxil) is a prodrug which means, it's an inactive compound that, once inside the body, is converted into the active beta-lactam antibiotic mecillinam. [1,2] It received Food and Drug Administration (FDA) authorization on 17 April 2024 to treat uncomplicated urinary tract infections (UTIs). [3,4] Pivmecillinam works against gram-negative bacteria and is similar to amdinocillin in its effects. [2]
Pharmacological Class: Penicillin class antibacterial [5]
Pivmecillinam (amdinocillin pivoxil) is a prodrug which means, it's an inactive compound that, once inside the body, is converted into the active beta-lactam antibiotic mecillinam. [1,2] It received Food and Drug Administration (FDA) authorization on 17 April 2024 to treat uncomplicated urinary tract infections (UTIs). [3,4] Pivmecillinam works against gram-negative bacteria and is similar to amdinocillin in its effects. [2]
Pharmacological Class: Penicillin class antibacterial [5]
Pivmecillinam is prescribed for the management of uncomplicated UTIs in women aged 18 and older, triggered by Escherichia coli (E. coli), Proteus mirabilis, and Staphylococcus saprophyticus. It should be used only for infections confirmed or likely caused by these bacteria to help prevent drug resistance and preserve the drug's effectiveness. [3]
Prodrug and Activation:
Pivmecillinam is a prodrug composed of the pivaloyloxymethylester of the amidinopenicillanic acid, mecillinam. It is well-absorbed after oral administration. Upon absorption, it is promptly hydrolyzed to mecillinam (active antibacterial drug) by non-specific esterases. These enzymes are found in the gastrointestinal mucosa, blood, and other tissues.
Beta-Lactam Activity:
Mecillinam is a beta-lactam antibiotic primarily active against gram-negative bacteria. It functions by hampering the formation of the bacterial cell wall.
Target and Specificity:
In contrast to most other beta-lactam agents, which primarily target penicillin-binding protein (PBP) -1A, -1B, or -3 in gram-negative bacteria, mecillinam exerts high specificity against PBP-2.
Action and Result:
By suppressing PBP-2, mecillinam disrupts bacterial cell wall formation. This disruption arouses cell lysis and bacterial death, especially in gram-negative organisms. [2, 5, 6]
Pivmecillinam's recommended dosage is 185 mg orally, three times a day for 3 to 7 days, based on clinical indication and severity of the infection. Administer pivmecillinam with or without food. [5]
Dosage modifications:
Special Note: If a dose of pivmecillinam is missed, patients must take it as soon as they recall. However, they should not double the dose to counterbalance the missed one. [5]
Absorption
Distribution
Metabolism
Excretion
Acute Porphyria
Pivmecillinam should not be used in porphyria-affected patients. This is because pivmecillinam has been strongly associated with precipitating acute and potentially severe porphyria attacks.
Carnitine Insufficiency
In those suffering from primary or secondary carnitine insufficiency stemming from inherited ailments of carnitine metabolism, mitochondrial fatty acid oxidation, and other inborn metabolic abnormalities (e.g., methylmalonic aciduria, propionic acidemia), pivmecillinam is not recommended.
Serious Hypersensitivity Reactions
Pivmecillinam is prohibited in individuals who have experienced a severe hypersensitivity reaction (e.g., anaphylaxis or Stevens-Johnson syndrome) to pivmecillinam or other beta-lactam antibacterial agents (such as cephalosporins and penicillins).[5]
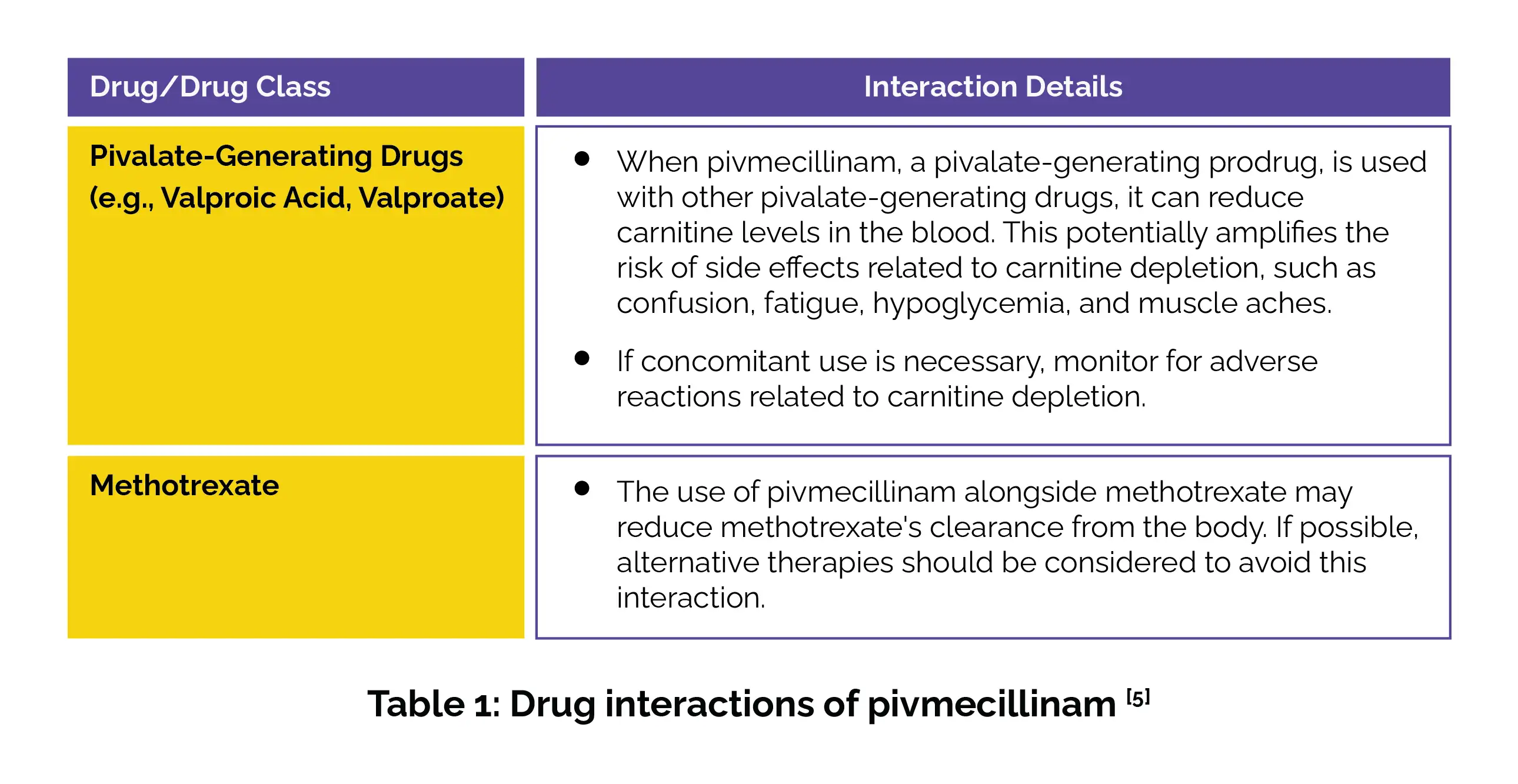
The drug interactions of pivmecillinam are summarized in Table 1:
The most commonly reported adverse effects (1-10%) are:
Hypersensitivity Reactions
Serious allergic reactions (e.g., anaphylaxis) may arise, especially in those with a history of penicillin, cephalosporin, or carbapenem allergies. So, before commencing pivmecillinam, examine for any history of hypersensitivity reactions. If an anaphylactic response occurs, discontinue its use and offer relevant therapy.
Risk of Drug-Resistant Bacteria
Avoid using pivmecillinam for conditions that are not proven or strongly suspected to be bacterial infections, or for prophylaxis, to prevent the development of drug-resistant bacteria.
Newborn Screening Interference
Administering pivmecillinam to pregnant women before delivery may result in a false positive for isovaleric acidemia in the newborn's screening. It is crucial to promptly follow up on any positive screening results to confirm the diagnosis.
Severe Cutaneous Adverse Reactions (SCAR)
Pivmecillinam has been linked to severe and potentially life-threatening skin reactions, including Acute Generalized Exanthematous Pustulosis, Drug Reaction with Eosinophilia and Systemic Symptoms, Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis. Vigilant monitoring is crucial—immediately discontinue pivmecillinam at the first indication of SCAR or any hypersensitivity symptoms.
Carnitine Insufficiency
Significant hypocarnitinemia has been observed in vulnerable patients, such as those with renal impairment, reduced muscle mass, or requiring long-term antibacterial therapy. Given this risk, pivmecillinam is not recommended for extended use. Additionally, evade co-administration with valproic acid, valproate, or other pivalate-releasing drugs, as these compounds heighten the risk of carnitine insufficiency. Alternative therapies should be considered for at-risk patients.
Clostridioides difficile-Associated Diarrhea (CDAD)
Almost all systemic antibacterials, including pivmecillinam, have been linked with CDAD, a potentially severe complication. Any patient experiencing diarrhea following pivmecillinam administration should be promptly evaluated for CDAD to ensure timely intervention. [5]
Pivmecillinam for Uncomplicated Acute Cystitis: A Review
A review by Burchette JE et al. investigated pivmecillinam for uncomplicated acute cystitis, focusing on its efficacy and safety. A comprehensive literature search, including PubMed and ClinicalTrials.gov, identified 6 randomized controlled trials (RCTs). As found, pivmecillinam was efficient at doses of 200 to 400 mg, taken 3 times daily for 3 to 7 days. The 400 mg dose and longer treatment durations illustrated a more consistent clinical and bacteriologic cure.
Pivmecillinam was more commonly found to be used outside U.S. FDA-approved populations, including men, pregnant individuals, and those with multidrug-resistant infections. Although data for pyelonephritis is limited, pivmecillinam shows promise as a potential treatment for acute cystitis, particularly as resistance to first-line therapies rises. Its use may be cost-dependent, but low resistance rates make it a valuable alternative when other treatments fail. [7]
Pivmecillinam as Oral Step-down Therapy for E. Coli Bacteremic UTI
According to the findings of a study, a regimen of oral pivmecillinam 400 mg 4 times daily for 1 week following 3 days of parenteral antibiotics is an effective and safe treatment option for patients with bacteremic UTI caused by E. coli. This study examined pivmecillinam as a step-down therapy for bacteremic UTI. After receiving 3 days of parenteral antibiotics, patients were transitioned to oral pivmecillinam (400 mg 4 times daily for 1 week). Of the 476 screened patients, 53 were included, with 50 evaluated in the per-protocol analysis.
The primary endpoint, a combination of afebrility, no need for retreatment, and improved health status, was achieved in 88% (44 out of 50) of patients. The test of cure (TOC) was carried out 1 week after treatment completion. However, 29.2% (14 out of 48) of patients exhibited substantial growth of E. coli on TOC, defined as greater than 103 colony-forming units/mL. Furthermore, C-reactive protein (CRP) levels were strongly associated with treatment outcomes (Odds ratio 0.006). [8]
Pivmecillinam for Uncomplicated Lower UTIs Caused by Staphylococcus saprophyticus
A study investigated pivmecillinam's efficiency in relieving uncomplicated lower UTIs triggered by Staphylococcus saprophyticus. Data were drawn from 4 RCTs where 74 UTI-affected females (aged 18–55 years; mean age 25 years) were empirically treated with pivmecillinam. The primary outcome, a cumulative clinical effect defined as symptom resolution within the first 8 days without recurrence at approximately 4 weeks, was attained in 72% (53 out of 74) of participants.
The secondary outcome, bacteriological effect (elimination of the causative pathogen in the initial follow-up urine sample), was observed in 86% (51 out of 59) of participants. Antibiotic susceptibility testing followed EUCAST standards, with significant bacteriuria defined as ≥10³ bacteria/mL. These findings highlight the high clinical and bacteriological efficacy of pivmecillinam against Staphylococcus saprophyticus-induced UTIs. [1]
Comments (0)